For many couples facing infertility, medicine offers a range of solutions. But for men with non-obstructive azoospermia (NOA)—a genetic condition where sperm production stalls—options remain limited.
Researchers at the University of Osaka in collaboration with Baylor College of Medicine have developed a pioneering approach to combat NOA. By delivering mRNA through lipid nanoparticles (LNPs) targeting specific testicular genes, they successfully restored sperm production and achieved the birth of viable offspring in a mouse model. This treatment led to healthy, fertile offspring and pointed toward realistic, gene-informed therapies.
The research is published in the journal Proceedings of the National Academy of Sciences.
Infertility affects one in six couples globally, with male factor infertility accounting for almost half of these cases. NOA, a condition characterized by the lack of sperm in the ejaculate despite normal hormonal levels, often stems from genetic defects disrupting spermatogenesis. Current treatments for NOA are limited, leaving thousands of affected individuals without viable options to conceive biologically.
The study focused on a NOA mouse model suffering from meiotic arrest due to a genetic deficiency. The team injected LNPs into the rete testis to fill seminiferous tubules and deliver mRNA broadly.
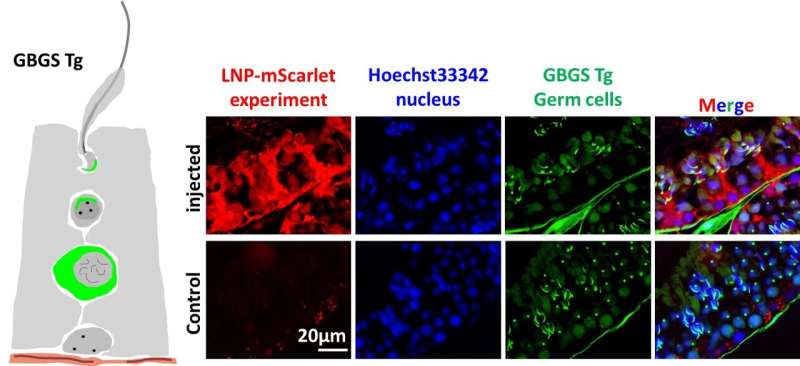
Expression lasted around five days and reached ~55% of tubules. To bias expression to germ cells (not Sertoli cells), they appended the Dsc1 3′-UTR containing a miR-471 target, shifting translation away from Sertoli cells and toward germ cells.
In Pdha2 knockout mice—where meiosis arrests—the delivered Pdha2 mRNA resumed meiotic progression, yielding round spermatids by two weeks and sperm by three weeks. Using testicular sperm for ICSI produced 26 pups from 117 embryos (22.2%), which developed normally, were fertile, and showed no large (>1 Mb) genomic alterations.
This study offers an approach for treating male infertility caused by genetic defects. By restoring spermatogenesis in a non-obstructive azoospermia (NOA) mouse model using lipid nanoparticle (LNP)-mediated mRNA delivery, it introduces a safer, non-integrating alternative to traditional gene therapies, providing hope for untreatable infertility cases in humans.
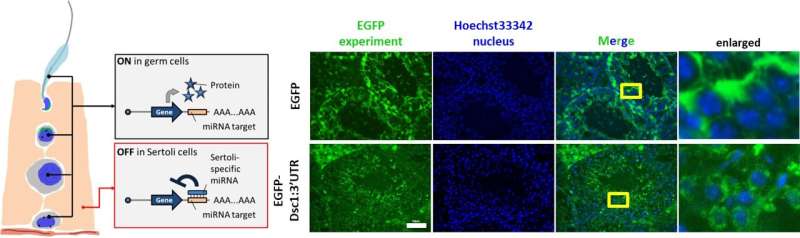
Professor Masahito Ikawa, senior author of the study says, “Using fully synthetic LNPs to deliver mRNA minimizes genome-integration concerns and enables us to restore spermatogenesis in a defined genetic model.”
Professor Martin M. Matzuk says, “These findings clarify how spermatogenesis can be rescued and lay the groundwork for applied research toward treating certain forms of male infertility.”
More information:
Ikawa, Masahito et al, Sperm and offspring production in a nonobstructive azoospermia mouse model via testicular mRNA delivery using lipid nanoparticles, Proceedings of the National Academy of Sciences (2025). DOI: 10.1073/pnas.2516573122. doi.org/10.1073/pnas.2516573122
Citation:
mRNA therapy restores sperm production and fertility in mice (2025, October 13)
retrieved 13 October 2025
from https://medicalxpress.com/news/2025-10-mrna-therapy-sperm-production-fertility.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.

