By stimulating macrophages—a type of white blood cell—with biological factors that mimic infection, researchers have uncovered genetic drivers of complex diseases such as inflammatory bowel disease (IBD), in one of the largest studies of its kind.
Published in Nature Communications, scientists from the Wellcome Sanger Institute reveal insights into how our genes influence the risk of developing common diseases, by studying immune cells that have been activated by 24 stimuli mimicking real-life conditions.
The findings have resulted in a large dataset known as MacroMap, which provides a new map of genetic effects in immune cells. The results also pave the way for future studies that aim to explore genes in certain disease contexts in a more realistic and representative way.
Many genetic variants—changes in DNA—associated with disease are thought to influence the extent to which genes are turned on or off. However, these changes are often missing from current databases that map genetic differences that influence gene expression. One reason for this is because studies often use ‘resting’ cells.
This means the cells have not been activated by different stimuli—for example, by bacterial or viral infections. This does not give the full picture of gene activity when cells are activated during a normal immune response.
In a new study, researchers from the Sanger Institute sought to understand how genetic differences between people affect the behavior of a type of immune cell, called a macrophage, when stimulated to different conditions and stressors. Macrophages are a form of white blood cell responsible for engulfing and digesting harmful substances or cellular debris.
Firstly, the researchers used human induced pluripotent stem cells (iPSCs), which are cells taken from adult tissue that can be programmed to become many different cell types. They took these stem cells from 209 healthy people and turned them into macrophages.
Then, to study how genes behave under different conditions, they activated the macrophages with 24 different stimuli that trigger an immune response and mimic infections and inflammation. These stimuli include components such as viral mimics, bacterial elements and immune signaling molecules.
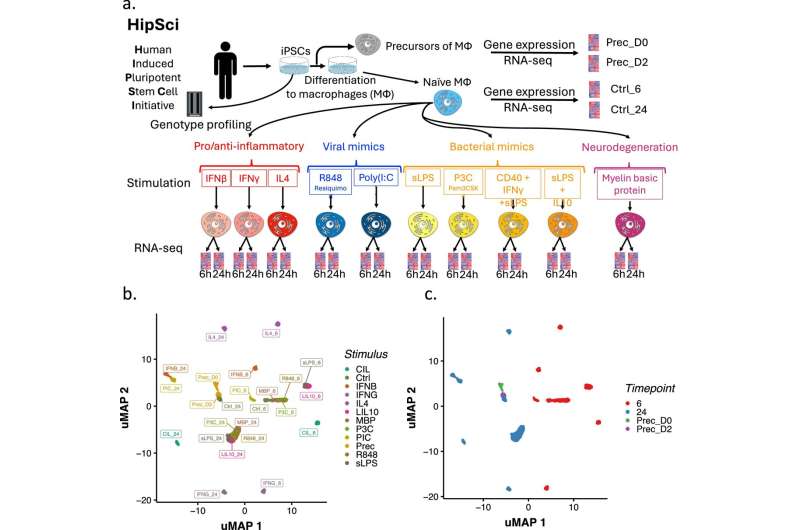
They then collected RNA from the cells six and 24 hours after stimulation to measure gene expression to see which genes were turned on or off in response to each stimulus.
The large-scale dataset produced from the study, called MacroMap, revealed that many links between genes and diseases were only visible after stimulation.
They found 1,955 instances where gene activity overlapped with genetic variants that are associated with disease, and over half of these—51%—would have been missed using unstimulated cells. For example, a genetic variant associated with coronary artery disease was found to increase the activity of a gene called CTSA, but only when macrophages were stimulated with inflammatory signals.
In a complementary study, which is also part of the MacroMap project and published in Nature Communications, the same team looked at RNA splicing—a process where cells cut and rearrange RNA, which is the instructions from DNA to make proteins.
The researchers aimed to understand how genes were spliced under the same 24 stimuli and how people’s genetic differences influenced splicing patterns.
They found that over 5,000 genes changed their splicing patterns when macrophages were activated by stimuli and that genetic risk factors for autoimmune diseases were linked to differences in splicing.
One genetic change was found to increase the use of a rare version of a gene called PTPN2, which normally helps control inflammation, and so it is suggested that this change may increase the risk of developing IBD.
Together, these studies highlight the importance of studying genes in the right biological context. What the researchers saw is that many disease-related genetic effects are invisible in resting cells, so future studies may increasingly focus on dynamic or stimulated cells to uncover the full picture of how genes influence health and disease.
By revealing how certain genes only become active during immune responses, this research may also inform treatment research such as RNA therapeutics, which offer a novel approach to targeting diseases that are difficult to treat with traditional medicines.
More information:
Nikolaos I. Panousis et al, Gene expression QTL mapping in stimulated iPSC-derived macrophages provides insights into common complex diseases, Nature Communications (2025). DOI: 10.1038/s41467-025-61670-9
Omar El Garwany et al, Splicing QTL mapping in stimulated macrophages associates low-usage splice junctions with immune-mediated disease risk, Nature Communications (2025). DOI: 10.1038/s41467-025-61669-2
Citation:
Activated immune cells reveal hidden drivers of autoimmune diseases (2025, August 27)
retrieved 27 August 2025
from https://medicalxpress.com/news/2025-08-immune-cells-reveal-hidden-drivers.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.

