The same technology behind MRI images of injury or disease also powers nuclear magnetic resonance (NMR) spectroscopy, which is used to analyze biological molecules for research on diseases and therapeutics. While NMR spectroscopy produces valuable data about the structure of molecules, the resolution is too low to sense individual atoms.
Now, quantum researchers at Purdue University are advancing an approach that could improve the resolution of NMR spectroscopy to the atomic scale and may also have applications in developing quantum computing and quantum communications.
“Conventional NMR spectroscopy is limited to measuring large samples of molecules. We’re interested in developing technologies that can detect and analyze a single molecule,” said Tongcang Li, professor of physics and astronomy in the College of Science and of electrical and computer engineering in the College of Engineering.
In research published in Nature, Li led a team that embedded the rare carbon 13 isotope in ultrathin hexagonal boron nitride, and then used magnetic resonance microscopy to obtain atomic-level information about the structure of the material they created.
Li is also a member of the Purdue Quantum Science and Engineering Institute, and director of the National Science Foundation’s Industry-University Cooperative Research Center for Quantum Technologies in Indiana. Working with Yuan Ping, an associate professor and theorist at the University of Wisconsin–Madison, they matched the information with predicted chemical structures.
Both MRI and NMR spectroscopy take advantage of the magnetic field that the nucleus of some atoms generate. The single proton inside a hydrogen nucleus, for example, generates a tiny magnetic field that is influenced by surrounding electrons and neighboring atoms. In MRI and NMR, the magnetic field of that nucleus is used to report on its environment.
In MRI, a powerful magnetic field aligns the hydrogen nuclei inside a patient’s body along a north-south pole, just as ordinary household magnets will align with one another. The exact energy required to flip each nucleus into the opposite orientation varies depending on the surrounding atoms and electrons.
When a radio wave of matching frequency is applied, the nucleus absorbs that energy and flips its orientation. As it relaxes back into alignment with the magnetic field, the nucleus emits a radio signal—with a frequency corresponding to the one it absorbed—that conveys information about its surroundings. During an MRI, a spectrum of different radio frequencies is pulsed through the body, and the returning signals are used to create detailed images of structures inside the body.
NMR spectroscopy uses a similar method to analyze milligram-sized samples of molecules.
Li’s lab is adapting the principle of magnetic resonance to a material that forms sheets of atoms only a few atoms thick, commonly called 2D materials. But in Li’s version of NMR, the imperfections he embedded in the 2D material—which are commonly called spin defects—will be used to report on the structure of a biological molecule placed on top of that material.
Because the molecule and the 2D material would be in close proximity, the atoms of the molecule would influence the spin defects embedded in the sheet, altering the signal that is returned during magnetic resonance. The result would be atomic-level information about the structure of the sample molecule.
To realize this vision, the researchers must first determine the structure and precisely control a single spin defect in the 2D material, in this case hexagonal boron nitride (hBN). As its name implies, hBN is formed of alternating boron and nitrogen atoms arranged in connecting six-sided rings. The lattice isn’t perfect, with occasional gaps, called vacancies, where a nitrogen or boron atom is missing from the lattice. If left empty, an electron settles in each vacancy.
In research published in 2022, Li’s team used the electrons in boron vacancies to control surrounding nitrogen nuclei and employed them as a quantum sensor. In that system, the electrons emitted light conveying information about the surrounding nitrogen nuclei.
But Li found that the electrons in the boron vacancy did not emit sufficient light to be seen individually. He used ensembles of thousands of boron vacancies to gather information with a resolution of 1 micrometer, which is far better than the 100-micrometer resolution of NMR, but still not the single-nucleus resolution that he wanted.
The discovery of carbon defects in hBN—without yet knowing the structure of those defects—offered a new opportunity. Ordinary carbon doesn’t generate a magnetic field and therefore can’t be used in magnetic resonance, so Li turned to carbon 13, a rare isotope with the usual six protons and an unusual seven neutrons in its nucleus. Carbon 13 does produce a magnetic field.
To make hBN with carbon 13 defects, Li’s team used a special carbon dioxide gas in which the carbon atoms are 99% carbon 13. By accelerating the atoms in the gas with an electric field, they essentially shot the atoms from the gas at a sample of hBN. Some carbon 13 and oxygen atoms displaced boron or nitrogen atoms in the crystal lattice of the hBN. Li’s team confirmed the location of the defects containing carbon 13 using optical microscopy.
But in a crystal lattice that could now host many possible combinations of boron, nitrogen, carbon 13 and oxygen, how could they determine the structure of those defects? To answer that question, Li’s team was able to use the carbon 13 nucleus as a probe.
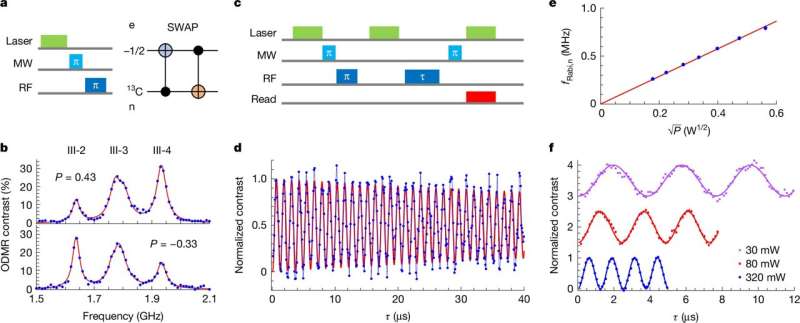
Using a third imaging technique based on the principle of magnetic resonance—optically detected nuclear magnetic resonance—they captured a signal that used the nucleus of the carbon 13 to report on the structure of its environment. Li said his work demonstrates the first single-spin NMR spectroscopy of a carbon 13 nuclear spin in a 2D material.
The team classified the defects into three groups based on the results. In collaboration with Ping, they identified the specific structure of defects in two of the groups. They also observed that the carbon 13 nuclear spin has a long coherence time, a characteristic which is advantageous in quantum computing applications, even at room temperature.
“This is the first time people used carbon 13 to create a spin defect in hexagonal boron nitride,” Li said. “Our work advances the understanding of spin defects in hexagonal boron nitride and provides a pathway to enhance quantum sensing with nuclear spins as quantum memories.”
More information:
Xingyu Gao et al, Single nuclear spin detection and control in a van der Waals material, Nature (2025). DOI: 10.1038/s41586-025-09258-7
Citation:
MRI technology inspires quantum advancement with 2D materials (2025, August 27)
retrieved 27 August 2025
from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.

