Carbon is the building block of biological life on Earth. The element is present in many compounds, such as sugars, proteins, and carbohydrate molecules, that comprise everything from animals to plants to bacteria.
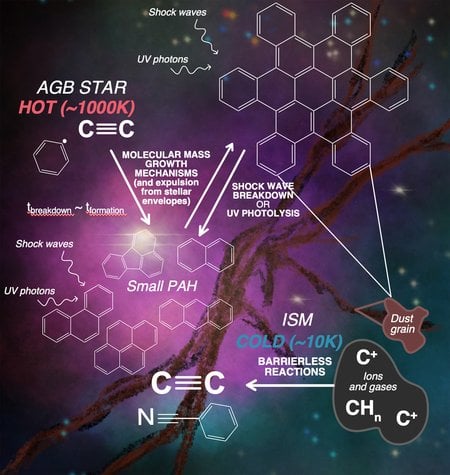
Schematic describing potential PAH formation processes: one around a hot star, and one in the cold interstellar medium. Image credit: S. Zeichner
One particular carbon-based molecule, called polycyclic aromatic hydrocarbons (PAHs), is both ubiquitous on Earth and abundant in space. Astronomers have observed signals of these honeycomb-shaped molecules in the space between stars, and estimate that PAHs constitute a significant percent of all carbon in the Milky Way galaxy and others. Understanding where and how astronomical PAHs form is crucial to understanding how this important biological building block found its way to Earth, but there are multiple theories regarding the environments that can form PAHs in space.
Now, a new Caltech-led study examining PAHs extracted from samples that were returned from the asteroid (162173) Ryugu finds the first evidence to support the theory that PAHs were formed within cold molecular clouds in interstellar space. The research required novel techniques in analytical chemistry developed at Caltech by postdoctoral scholar Sarah Zeichner (MS ’20, PhD ’24), the study’s first author.
The work was primarily conducted in the laboratory of John Eiler, the Robert P. Sharp Professor of Geology and Geochemistry. A paper describing the research appears in the issue of the journal Science.
Molecular clouds are very cold regions of gas in interstellar space, billions of miles across. Their frigid temperatures—which get down to -440 degrees Fahrenheit—mean that there is not much energy to generate chemical reactions. PAHs are relatively large molecules, so if they do form within cold molecular clouds, as some theories have suggested, their formation must occur through chemical reactions that require minimal thermal energy or are helped by absorbing light energy.
Most carbon on Earth consists of the common isotope carbon-12, the nucleus of which contains six protons and six neutrons. However, a small fraction of Earth’s carbon has seven instead of six neutrons in its nucleus, and is known as carbon-13. The extra neutron makes carbon-13 heavier than carbon-12; importantly, when chemical bonds are formed with carbon-13, the overall energy of the molecular system is lowered, which is preferred in cold, low-energy environments such as molecular clouds. Likewise, two carbon-13 atoms bonded together, creating so-called isotopic “clumps,” are even more energetically preferred in such environments. Thus, if PAHs were formed in a low-energy environment like a cold molecular cloud, researchers would expect to see an excess of carbon-13 clumps within them, as compared with the amount of clumps that are expected for PAHs that formed within high-temperature, high-energy environments such as the regions around stars.
In 2020, the Japanese space agency’s Hayabusa2 mission returned five grams of samples from the asteroid Ryugu. Seven milligrams of these samples were allocated to the study of carbon-containing molecules, also known as organic molecules. After various compounds were extracted and shipped to various teams around the world, an even smaller amount remained for studying the isotopic contents of PAHs.
“There were literally only six drops left,” says Zeichner. “And within these few drops, the concentrations of PAHs were 1,000 times less than what you need for traditional isotope analysis. So, I developed a unique method for this study. It uses the Orbitrap, an emerging technology in the study of isotopic properties, and tailors methods we had recently developed to be able to observe the isotopic properties of PAHs in very low abundance.”
Using these new analytical tools, Zeichner and her team determined that the PAHs isolated from the asteroid contained excesses in clumps of carbon-13, providing the first quantitative evidence that PAHs may have formed in the cold, low-energy regions of interstellar space.
The asteroid Ryugu belongs to a particular class of space rocks that are representative of the composition of an average solar system, and thus gives clues to the environment in which Earth and other planets of our solar system formed, and also, possibly, those of planets around other stars resembling our sun.
“Sample-return missions are broadly important for studying organic compounds,” says Zeichner. “Meteorites bring samples of space to Earth, but because they fall through the atmosphere, they undergo some terrestrial contamination. These special samples offer an opportunity to look at organic molecules that have never experienced uncontrolled exposure to the terrestrial biosphere. And, hopefully, these methods can now help us better understand samples from meteorites and other extraterrestrial bodies that are returned by future missions.”
Written by Lori Dajose
Source: Caltech

