Filoviruses are among the globe’s most lethal—indeed, so dangerous they can be handled only in high-security laboratories. Yet, more than five decades after the discovery of the Marburg virus and nearly 50 years after the first outbreak involving its infamous cousin—the Ebola virus—questions still abound about the family Filoviridae.
Members of this deadly family emerged through zoonotic transmission, most likely jumping across multiple species barriers—from fruit bats to simian species and forest antelopes, then eventually to humans. It’s theorized that humans may have contracted the viruses through hunting and butchering bushmeat.
Mindful that only two vaccines exist, and both were designed to prevent Ebola virus infection, a multidisciplinary team of scientists at a U.S. military research institute has analyzed whether one of those vaccines might also confer immunity to other filoviruses. The research arrives as virologists at the University of Texas Medical Branch in Galveston are testing the antiviral pill known as obeldesivir, which so far appears capable of curing Rhesus macaques infected with Ebola virus. The medication is the oral form of the intravenous drug Remdesivir, originally developed for COVID-19.
Military researchers, meanwhile, conducted an in-depth series of experiments to determine whether a recombinant Ebola vaccine containing components of multiple filoviruses could serve as an off-the-shelf choice to prevent infection by filoviruses other than Ebola.
Their findings, however, didn’t produce the outcome they had hoped for. But the discovery that unfolded has helped pave the way to a broader understanding of what’s needed for a multivalent vaccine that guards against multiple filoviruses. Details of the research are reported in the journal Science Translational Medicine.
“Ebola virus is a negative single-strand RNA virus from the family Filoviridae that causes a virulent hemorrhagic fever, termed Ebola virus disease,” writes Dr. Thembi Mdluli, lead author of the new research and a computational biologist at Walter Reed Army Institute of Research in Silver Spring, Maryland.
Just as Ebolavirus is a negative single-strand RNA virus, so too are other filoviruses, which also cause a devastating hemorrhagic fever. Filoviruses are known for their morbidity and high mortality. Mduli and colleagues pointed to Marburg outbreaks in Africa, where fatality rates have ranged between 83 and 88%.
“Marburg virus—MARV—was discovered in 1967 and has caused mostly sporadic self-contained outbreaks,” Mdluli added, referring to the world’s first filovirus discovery. The isolation of the Marburg virus occurred after lab personnel in Europe were infected by green monkeys from Uganda. Laboratory workers were infected in Marburg and Frankfurt, Germany, as well as in Belgrade, Serbia. Of the 31 people who were sickened, seven died.
In addition to Ebola and Marburg viruses, other members of the filovirus family include Sudan, Bundibugyo and Tai Forest viruses, all named after geographic locations where they were first identified. The Ebola virus is named after the Ebola River in the Democratic Republic of the Congo. Its first known appearance was an explosive and deadly August 1976 outbreak. An estimated 280 people died.
The Walter Reed researchers report that a vaccine to prevent infections caused by filoviruses is critical. Likewise, virologists in Texas, working on the obeldesivir antiviral research, say a single pill would solve a global health problem.
“We’re really trying to come up with something that is more practical, easier to use, that could be used to help prevent, control, and contain outbreaks,” Thomas Geisbert, a virologist at the University of Texas Medical Branch, told Agence France Presse in March.
Despite the amount of time that filoviruses have circulated on Earth, they emerged only in the 20th and 21st centuries. It has taken until recent years to develop vaccines and monoclonal antibody therapies just for Ebola virus disease.
“Filoviruses are ancient relative to their initial detection in humans. The most recent common ancestor of Filoviridae is estimated to be several thousand years old,” Mdluli asserted.
It was in the aftermath of the sweeping 2014 through 2016 West African Ebola outbreak that vaccine-development studies produced the first Ebola inoculation, Ervebo, which was approved by the U.S. Food and Drug Administration and the European Medicines Agency in 2019. It’s manufactured by Merck & Co. Vaccine candidates are in development for Marburg virus, but there still isn’t an approved immunization to prevent infection.
Mdluli and her colleagues chose to study the only other approved Ebola vaccine, one developed by Belgium-based Janssen Pharmaceuticals, a division of Johnson & Johnson. The Janssen Ebola vaccine is a two-part regimen known as Zabdeno and Mvabea. The doses were designed to be administered eight weeks apart.
The first part of the vaccine—the Zabdeno dose—includes an adenovirus vector that ferries the vaccine’s payload—the genetic code for Ebola virus’s sugary outer coating, its glycoprotein—into human cells.
The second dose consists of a modified vaccinia Ankara vector, the vehicle that carries the vaccine into cells. It encodes glycoproteins from Ebola, Sudan and Marburg viruses, as well as the nucleoprotein from the Tai Forest virus. In the study, the two vaccines were administered to 583 people.
Volunteers hailed from multiple countries: Kenya, Mozambique, Nigeria, Tanzania, Uganda, and the United States. Seventy-one participants were from the U.S.
Volunteers were divided into two groups. This allowed the team to vaccinate some participants in the manufacturer’s prescribed order for the two doses: Zabdeno first and Mvabea second.
With the second group, the team reversed the order of the vaccines: Mvabea first, followed by Zabdeno, providing exposure to the components of multiple filoviruses in participants’ initial shots. Researchers also studied 48 people who were infected during an outbreak of Bundibugyo virus, a filovirus that emerged in Uganda in 2007.
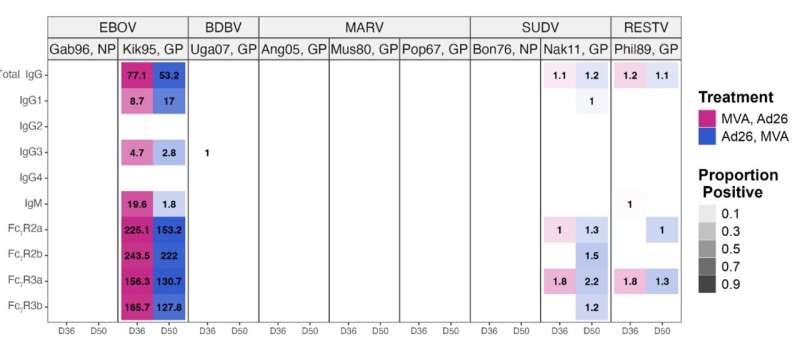
When participants’ blood was analyzed in the lab, it revealed that antibodies were highly specific to fighting Ebola virus but didn’t react strongly to other filoviruses. The scientists also examined blood samples from the Bundibugyo survivors and discovered their antibodies didn’t put up much of a fight against other filoviruses, either.
Mdluli reported that her team’s strategy provided only limited protection against other members of the filovirus family.
“These results underscore the vaccine’s potential to provide strong, species-specific protection against [Ebola virus] while highlighting the need for multivalent vaccines that can provide broader coverage for other filoviruses,” Mdluli concluded.
More information:
Thembi Mdluli et al, Ebola virus vaccination elicits Ebola virus–specific immune responses without substantial cross-reactivity to other filoviruses, Science Translational Medicine (2025). DOI: 10.1126/scitranslmed.adq2496
© 2025 Science X Network
Citation:
Scientists work toward a vaccine for filoviruses, Ebola’s deadly kin (2025, April 27)
retrieved 27 April 2025
from https://medicalxpress.com/news/2025-04-scientists-vaccine-filoviruses-ebola-deadly.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.

