A team of researchers led by Dr. Marcus Conrad from Helmholtz Munich has discovered a novel anti-cancer drug called icFSP1, which sensitizes cancer cells to ferroptosis. The results of this research are published in Nature.
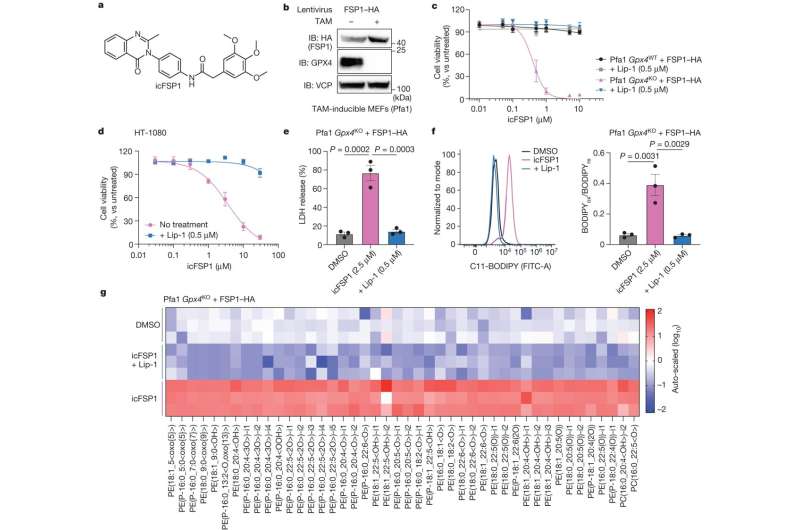
Ferroptosis is characterized by the iron-dependent oxidative destruction of cellular membranes, which is counteracted by ferroptosis suppressor protein-1 (FSP1), one of the guardians of ferroptosis. Although FSP1 has been considered as an attractive drug target for cancer therapy, in vivo efficacious FSP1 inhibitors have been lacking. To this end, the team carefully evaluated hits from a screen of approximately 10,000 small molecule compounds, and identified icFSP1 as a new in vivo effective drug.
Importantly, the team uncovered the mechanism-of-action of icFSP1, which is based on triggering phase separation of FSP1, a physical phenomenon in cells similar to the separation of oil and water. In fact, icFSP1 strongly inhibited tumor growth in vivo with distinct condensates of FSP1 in tumor tissue, thus proposing a new concept to combat tumors by promoting phase separation of FSP1 and ferroptosis.
icFSP1 is an in vivo applicable FSP1 inhibitor
Resistance to chemotherapy or metastasis is an important clinical problem in cancer. Notably, during de-differentiation or metabolic rewiring, certain malignant cancer cells acquire an intrinsic vulnerability to ferroptosis. Therefore, targeting ferroptosis has shown great promise as an approach to cancer therapy. In 2019, a team of researchers working with Marcus Conrad, Director of the Institute of Metabolism and Cell Death at Helmholtz Munich, had already identified the first FSP1-specific inhibitor, known as iFSP1.
“However, this compound is not suitable for in vivo use and exhibits off-target effects at high concentrations,” Conrad explained. To identify in vivo active ferroptosis inducers targeting FSP1, the team performed a screening campaign in cells, followed by DMPK (“drug metabolism and pharmacokinetics”) validation studies. These efforts eventually led to the identification of icFSP1 as a novel class of compounds that sensitize many human cancer cells to ferroptosis and attenuate tumor growth in vivo.
icFSP1 triggers phase separation of FSP1
By investigating the underlying mechanism-of-action (MoA) of icFSP1 in detail, the team further showed that icFSP1 does not inhibit FSP1 enzyme activity directly, but rather triggers the subcellular relocalization of FSP1 in a process known as phase separation. These results are in contrast to the MoA of the first FSP1 inhibitor iFSP1.
Toshitaka Nakamura, lead author of the study, further explains that “icFSP1-induced FSP1 condensations require distinct structural components and N-terminal myristoylation,” as demonstrated by using recombinant FSP1 proteins as well as different cell lines and tumor samples.
Breakthrough: Linking ferroptosis and phase separation
Ferroptosis has attracted overwhelming interest in many fields including cancer, neurodegenerative diseases, and ischemia/reperfusion injury similar to the process of phase separation, a fundamental physical phenomenon underlying signal transduction and basic biology related to multiple diseases. This study unravels for the first time a link between ferroptosis and phase separation.
“Our study will therefore serve as a good model for the further development of innovative therapeutic approaches in certain diseases, including cancer, in which ferroptosis/phase separation plays an important role,” explains Conrad, providing a promising outlook for the future.
More information:
Toshitaka Nakamura et al, Phase separation of FSP1 promotes ferroptosis, Nature (2023). DOI: 10.1038/s41586-023-06255-6
Citation:
Team introduces new approach in cancer therapy with innovative mechanism-of-action for ferroptosis induction (2023, June 28)
retrieved 28 June 2023
from https://medicalxpress.com/news/2023-06-team-approach-cancer-therapy-mechanism-of-action.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.

