
Growing up in Brazil, Marcos Simões-Costa often visited his grandparents’ farm in the Amazon. That immersion in nature — squawking toucans and all — sparked his fascination with science and evolution. But a video of a developing embryo, shown in his middle school science class, cemented his desire to become a developmental biologist.
“It’s such a beautiful process,” he says. “I was always into drawing and art, and it was very visual — the shapes of the embryo changing, the fact that you start with one cell and the complexity is increasing. I just got lost in that video.”
Today, Simões-Costa, of Harvard Medical School and Boston Children’s Hospital, is honoring his younger self by demystifying how the embryo develops. He studies the embryos and stem cells of birds and mice to learn how networks of genes and the elements that control them influence the identity of cells. The work could lead to new treatments for various diseases, including cancer.
“The embryo is our best teacher,” he says.

Sign Up For the Latest from Science News
Headlines and summaries of the latest Science News articles, delivered to your inbox
Client key* E-mail Address* Go
Thank you for signing up!
There was a problem signing you up.
Standout research
Simões-Costa focuses on the embryo’s neural crest cells, a population of stem cells that form in the developing central nervous system. The cells migrate to other parts of the embryo and give rise to many different cell types, from the bone cells of the face to muscle cells to brain and nerve cells.
Scientists have wondered for years why, despite being so similar, neural crest cells in the cranial region of the embryo can form bone and cartilage, while those in the trunk region can’t form either. While a postdoc at Caltech, Simões-Costa studied the cascade of molecules that govern how genes are expressed in each cell type. With his adviser, developmental biologist Marianne Bronner, he identified transcription factors — proteins that can turn genes on and off — that were present only in cranial cells. Transplanting the genes for those proteins into trunk cells endowed the cells with the ability to create cartilage and bone.
Now in his own lab, he continues to piece together just how this vast regulatory network influences the specialization of cells. His team reconstructed how neural crest cells’ full set of genetic instructions, or the genome, folds into a compact, 3-D shape. The researchers identified short DNA sequences, called enhancers, that are located in faraway regions of the genome, but end up close to key genes when the genome folds. These enhancers work with transcription factors and other regulatory elements to control gene activity.
Simões-Costa is also using neural crest cells to elucidate a strange behavior shared by cancer cells and some embryonic cells. These cells produce energy anaerobically, without oxygen, even when oxygen is present. Called the Warburg effect, this metabolic process has been studied extensively in cancer cells, but its function remained unclear.
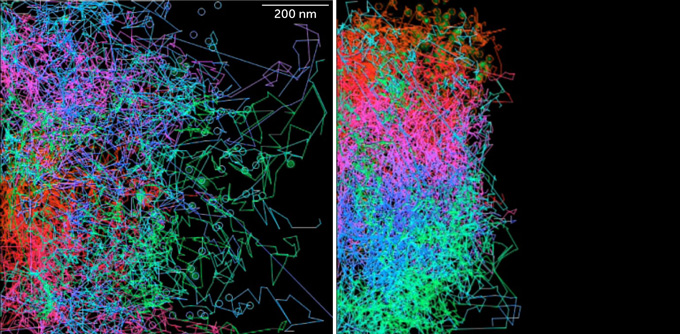 When neural crest cells are soaked with a drug that forces them to metabolize oxygen, they don’t move as much (right) as cells that aren’t treated (left). Cell movements are tracked over 12 hours. D. Bhattacharya, A.P. Azambuja, M. Simões-Costa/Developmental Cell 2020
When neural crest cells are soaked with a drug that forces them to metabolize oxygen, they don’t move as much (right) as cells that aren’t treated (left). Cell movements are tracked over 12 hours. D. Bhattacharya, A.P. Azambuja, M. Simões-Costa/Developmental Cell 2020 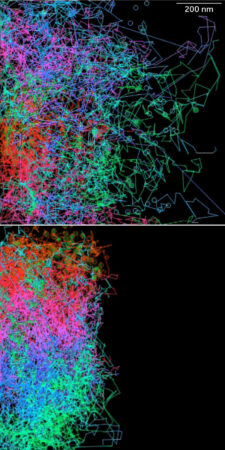 When neural crest cells are soaked with a drug that forces them to metabolize oxygen, they don’t move as much (bottom) as cells that aren’t treated (top). Cell movements are tracked over 12 hours. D. Bhattacharya, A.P. Azambuja, M. Simões-Costa/Developmental Cell 2020
When neural crest cells are soaked with a drug that forces them to metabolize oxygen, they don’t move as much (bottom) as cells that aren’t treated (top). Cell movements are tracked over 12 hours. D. Bhattacharya, A.P. Azambuja, M. Simões-Costa/Developmental Cell 2020
Through experiments manipulating the metabolism of neural crest cells, Simões-Costa’s team found that the Warburg effect is necessary for the cells to move around during early development. The mechanism, which should stay turned off in nonembryonic cells, somehow “gets reactivated in adult cells in the context of cancer, leading those cells to become more migratory and more invasive,” Simões-Costa says.
“He’s one of the few people who’s really looked at [this process in neural crest cells] at a molecular level and done a deep dive into the mechanisms underlying it,” says Bronner.
Cleverly combining classical embryological methods with the latest genomic technologies to address fundamental questions in developmental biology is what makes Simões-Costa special, says Kelly Liu, a developmental biologist at Cornell University. He wants to understand not only what individual genes do, but how they work at a systems level, she says.
What’s next
How does the genetic blueprint tell cells where they are in the embryo, and what they should be doing? How do cancer cells hijack the Warburg effect, and could understanding of that process lead to new treatments? These are some of the questions Simões-Costa wants to tackle next.
“It’s been 20 years since the Human Genome Project came to a conclusion,” he says, referring to the massive effort to read the human genetic instruction book. “But there’s still so much mystery in the genetic code.”
Those mysteries, plus a deep passion for lab work, fuel Simões-Costa’s research. “Being at the bench is when I’m the happiest,” he says. He likens the delicate craft of performing precise surgeries on tissues and cells to meditation. “It does not get old.”
Want to nominate someone for the next SN 10 list? Send their name, affiliation and a few sentences about them and their work to [email protected].

