It’s rare that compelling clues converge to illuminate a longstanding medical mystery: the origin of the Black Death, a bubonic plague that killed an estimated 25 million people in Europe almost seven centuries ago. Tombstones near the Tian Shan mountains in Kyrgyzstan bearing dates and the word “pestilence” and DNA from plague bacteria in the remains beneath them now place a date — 1338 — to an event that could have kicked off the plague 8 years later.
Perfect storm
“Just like COVID, the Black Death was an emerging disease, and the start of a huge pandemic that went on for some 500 years,” said Johannes Krause, director of the department of archaeogenetics at the Max Planck Institute for Evolutionary Anthropology in Leipzig, during a webinar to accompany publication of the findings in Nature. “It’s very important to understand the circumstances in which it emerged.”
Krause described the origin of what’s known as the second pandemic of the Black Death as “a chance event that came to Europe and found perfect conditions — a naïve population of black rats and human populations that had not experienced plague for 600 years [since the first plague in 6th century Europe]. No one had immunity. Those were the conditions of a perfect storm.”
Academics have puzzled over its site of origin for two centuries. East or central Asia? Between the Black and Caspian seas? In India, Mongolia, the Urals, or western Siberia?
Wherever the disease began, took hold, and then spread, the tentacles of the Black Death ultimately claimed nearly 60% of the population of western Eurasia between 1346 and 1352.
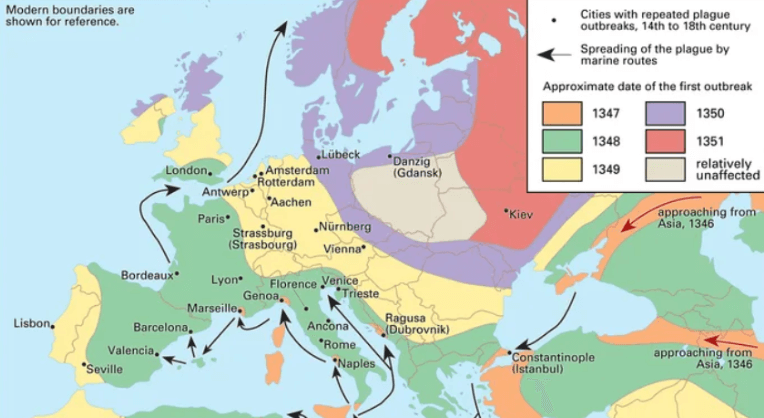
Once genome sequences of the bacterial pathogen, Yersinia pestis, became available starting in 2001, researchers were able to compare strains to hypothesize origins, but only as far back as “a star-like emergence of four major lineages,” they write. That’s like starting a novel at the third chapter.
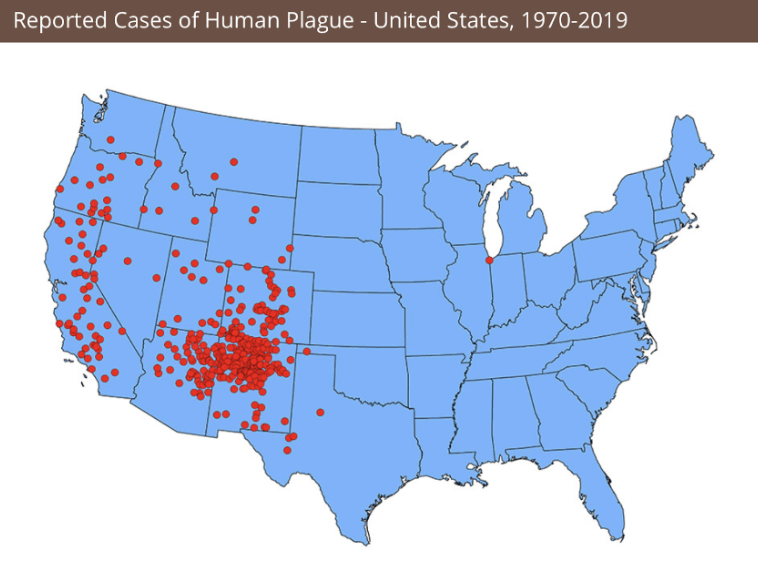
Remnants of plague persist in rodent populations in Eurasia, Africa and the Americas. Worldwide, 1,000 to 2,000 cases are reported each year, with only on average 7 in the US, usually in rural areas where rats wander indoors. That happens in northern New Mexico and Arizona, southern Colorado, California, southern Oregon, and far western Nevada. In the west, prairie dogs carry the infection.
Plague primer
Yersinia pestis evolved 1,500 to 20,000 years ago from a more benign human gut pathogen, Yersinia pseudotuberculosis.
The plague bacterium has an unusually dynamic genome, with genes added, moved, and silenced over the millennia. “This flexibility in its genome has made Yersinia pestis a finely tuned pathogen that is adaptable to new routes of transmission to humans,” Brendan Wren, from the London School of Hygiene and Tropical Medicine, told me when I wrote about sequencing its genome in 2001 for The Scientist. Genetic diversification gave rise to the Black Death strain from bacteria already in the area.
The bacteria wreak quick havoc on a human host. Fleas pick up bacteria from infected rats or other small mammals. The microbes reproduce so rapidly that they block the insect’s gut. Starving, the flea goes into a feeding frenzy. But the rats are dying too fast, so the fleas attack people, a single bite delivering about 24,000 bacteria.
“Humans are collateral damage of a rodent disease. The fleas are looking for the next warm body. They don’t really like us, but if no rodents are around, they’ll bite a human,” said co-author Philip Slavin, from the University of Stirling in Scotland.
The bacteria head for the lymph nodes, where they proliferate. Two to six days later, headache and fever start. In another eight hours, the characteristic black “buboes” appear, which are agonizing swellings near the lymph nodes. The bacteria then release a toxin that causes circulatory collapse, leading quickly to shock and organ failure. Meanwhile, the bacteria also disable much of the immune response.
Death comes in days from the highly coordinated attack. In the rarer pneumonic plague, the infection spreads person-to-person through the air. It hasn’t been seen since before the last century.
The earliest known description of bubonic plague comes from Greek physician Rufus of Ephesus, who practiced from 98-117 AD and wrote,
The buboes called pestilential are most fatal and acute, especially those that are seen occurring about Libya, Egypt, and Syria.
Plague came to the U.S. in the 1900s on steamships teeming with rats, mostly from Asia. The most recent urban epidemic of plague was in Los Angeles in 1924 and 1925, when city rats spread the bacteria to rural rats.

How did the Black Death begin?
The geographical distribution of modern cases suggests an initial event between western Eurasia and eastern Asia. Until now, historical and archaeological evidence has dominated investigations and interpretations, with ancient DNA filling in only a few blanks. As in other fields, an historic Eurocentric focus has obscured crucial details, telling only part of a story.

Philip Slavin, who describes himself as an environmental and ecological historian, is interested in the global history of epidemics. “I’ve always been fascinated with the Black Death. One of my dreams was to actually be able to solve this riddle of its origins,” he said.
In 2017, he learned of two Christian cemeteries in the Chüy Valley near Lake Issyk-Kul in modern Kyrgyzstan. From 1885 to 1892, archaeologists had discovered 30 skeletons there that provided evidence of an epidemic during the fourteenth century.

The remains ended up at the Peter the Great Museum of Anthropology and Ethnography in St. Petersburg, Russia. The investigators got permission to sample DNA from the skeletons.
The DNA held quite a surprise.
“Out of 467 tombstones that are precisely dated to the period from 1338 to 1343, 118 are dated to 1338 and 1339 — that’s a huge spike,” Slavin said.
The short inscriptions were in Syriac, a western dialect of Aramaic that was the language of ancient Syria and early Christian texts.

“Ten stones from 1338 and 1339 had inscriptions that said the person buried there died of pestilence. That was exciting! Because one or two years with excess mortality means something funny was going on,” Slavin added.
Was the new evidence marking the dawn of the second plague pandemic? The “something funny going on” was that the two-year span, 1338 to 1339, was 7 or 8 years before the Black Death came to Europe. In addition, artifacts and coins found in the graveyard, like crime scene evidence, and historical records indicate widespread trade practices that could have spread the pestilence. The site had pearls from the Pacific or Indian oceans, corals from the Mediterranean, and coins minted in Iran and Afghanistan.
Slavin assembled the clues.
A picture emerges of a community situated at the heart of long-distance inland trade routes, the silk roads. This was a rest stop, perhaps. Just over 1000 people lived there, on the eve of the plague outbreak. Hundreds of urban settlements towards the Black Sea were active in international trade and regional exchange of goods. Trade played a massively important role in spreading plague westward.
But the absence of written sources from central Asia from that time stymied efforts to link the human remains with the start of the Black Death. That’s what drove Slavin to contact colleagues at the Max Planck Institute, where researchers analyze genes and genomes of human ancestors.
DNA evidence
To complement the clues from classical archaeology, Maria Spyrou, a postdoctoral researcher at the University of Tubingen and first author of the Nature report, and colleagues analyzed DNA from the remains for more than a million genetic markers — single-base signposts (SNPs) in the genome that vary in populations and are used to trace ancestry.
“Despite the risk of environmental contamination and no guarantee that the bacteria would have been able to be preserved, we analyzed DNA from seven individuals from two cemeteries. We found ancient DNA of the plague bacterium in three individuals,” Spyrou said. The tombstones of the trio bore the death date of 1338.
The researchers collected DNA from teeth, which are not only more resistant to environmental damage than bone but can harbor DNA evidence of past infections in what was once a blood supply. “So when we drill into teeth, into the pulp chamber, there’s a high chance of detecting (DNA from) bloodborne infections that affected individuals at the time of death,” Spyrou explained.
Computational techniques then compared DNA patterns from the teeth to a database of thousands of microbial genomes. One hit was Yersinia pestis.
Spyrou knew the DNA was ancient, and not contemporary contamination, from a telltale pattern of damage “indicating that this was an infection at the time of death. We also reconstructed the entire genome of the bacteria, which revealed that it is ancestral to all modern strains that have since diverged.”
The technology to analyze ancient DNA has galloped forward since the first plague genome was sequenced. Said Krause, “The DNA was highly degraded, broken into pieces averaging 50 base pairs of the 4.2 million in the genome. Reconstructing ancient genomes from millions of little pieces is now an established method.”
Geneticists compare DNA sequences to deduce relationships. The closer the sequences among pairs of individuals, the more closely related they are in an evolutionary sense — that is, how much time has passed since they shared an ancestor. It’s the same logic behind consumer DNA testing that ranks the closeness of relatives and tracking the evolution of new viral variants. Researchers use mutation rates for specific genes to date ancient DNA.
For the tombstones, the DNA data jibe with the death dates, Spyrou concluded.
The epidemic of 1338 was caused by a strain of the bacterium that is a direct ancestor of the strain that 8 years later caused the Black Death of Europe. The first wave began in 1346 in the Black Sea and killed half the population of Europe within the next decade. This result and other analyses led us to conclude that the location and time when plague emerged before causing the Black Death was most likely central Asia and likely happened during the first half of the 14th century.
That might have been at or within a few kilometers of the graveyard.
Bringing the story into the present was telling, too. “We found not just the ancestor of the Black Death pathogen, but the ancestor of the majority of plague strains circulating today,” Krause said. “This burial ground, close to the capitol of Kyrgyzstan, is the ancestor of 4 of the 5 lineages, so it is the big bang of plague. The cemetery holds the most recent common ancestor. A flower bouquet of strains was born at that time.
Additional evidence comes from the DNA of other small mammals living in the area, especially marmots, a type of large ground squirrel. “Marmots of that region today carry the closest living relatives to the original plague strain that gave rise to all others, including the one that caused the Black Death,” Krause said. Spyrou added that perhaps marmots facilitated a spillover event that led to the 1338 outbreak.
If the spillover to humans was the ignition of the Black Death, then human behavior was the accelerant.
Is a plague epidemic possible today?
Given COVID, we’re all more sensitive to the continuing threat of infectious diseases. Could small rodents ever bring back plague? That’s unlikely, for a few reasons.
First, we have antibiotics.
Second, bubonic plague requires an intimate relationship with rodents. It doesn’t spread like SARS-CoV-2 in a subway car filled with coughing commuters. Improvement in human hygiene has distanced us from rats.
Third, the black rat that harbored Y. pestis during the Black Death was replaced in the 18th century by the brown rat, which is the most common rat today. It’s less likely to carry plague.
“Our study puts to rest a centuries-old controversy and determines when and where the single most notorious killer of humans began,” said Slavin. Perhaps one day researchers will knit similar strands of evidence into a tapestry that reveals the origin, or origins, of COVID.
Ricki Lewis, PhD is a writer for PLOS and author of the book “The Forever Fix: Gene Therapy and the Boy Who Saved It.” You can check out Ricki’s website and follow Ricki on X @rickilewis
This article previously appeared on the GLP on June 21, 2022.

