In November, a new coronavirus variant took the world by storm. Omicron has since caused an unprecedented wave of infections, striking about 90 million people in just 10 weeks. That’s more COVID-19 cases than were recorded in all of 2020.
Omicron also left scientists scratching their heads. It’s riddled with mutations, which might normally doom a virus. Early experiments showed that omicron wasn’t nearly as good as the previous coronavirus variant champ, delta, at melding with a cell’s membrane — crucial for infecting that cell — or at replicating in lung cells. Yet here it was, sweeping delta virtually off the map in just weeks in some places (SN: 2/10/22). Omicron even managed to infect people who already had immunity to the virus from vaccines or previous cases of COVID-19. How, researchers wondered, was omicron doing it?
“It’s a very interesting variant,” says virologist Shan-Lu Liu, who codirects the Viruses and Emerging Pathogens Program at The Ohio State University in Columbus. “I call it weird.”
Sign up for e-mail updates on the latest coronavirus news and research
Researchers in Botswana and South Africa were the first to unveil omicron’s genetic makeup. Their analysis revealed more than 60 mutations, including 42 changes alone in omicron’s spike protein — the knobby structure on the surface of the virus that initiates a cell break-in and can help evade antibody defenses. Some of those mutations have popped up in previous variants, including alpha and delta. But omicron has never-before-seen tweaks and unique combinations of mutations.
Scientists have been scrambling to discover how those changes affect omicron’s ability to infect people and cause disease. Researchers around the world are infecting cells in lab dishes with omicron mimics, putting the virus under the microscope, testing the viral variant in lab animals and examining medical and other records all to discover what makes the variant tick. Here’s what scientists have found so far.
1. Omicron keeps it together.
The secret of omicron’s increased transmissibility has proven elusive. The spike protein is certainly one key to its success, allowing omicron to infect nearly 10 times as many cells as earlier versions of the virus, researchers in China reported December 17 in Signal Transduction and Targeted Therapy.
It is “distinct from all other variants, from a structural point of view,” says structural biologist Priyamvada Acharya. She and her colleagues at the Duke Human Vaccine Institute in Durham, N.C., used cryo-electron microscopy and other techniques to examine omicron’s spike protein.
In past studies, researchers, including Acharya, have pinpointed how certain mutations have given previous variants a leg up. For instance, some mutations in the delta variant’s spike protein helped that version of the coronavirus more easily grab a human protein called ACE2 or more readily fuse with human cells (SN: 12/16/21). Omicron shares some of those mutations but has many others that drastically change how the variant behaves. “It’s not just one thing,” Acharya says. “It’s multiple properties of the spike that confer an advantage to omicron.”
Like other versions of the coronavirus, each of omicron’s knobby spikes consists of three identical pieces that snap together in a single unit. Each of those pieces has a jointed, fingerlike portion called the receptor binding domain that reaches out, much like the prongs of a claw machine, to grasp ACE2 and anchor the virus to the cell it will infect.
Many antibodies that prevent the virus from entering cells target those fingers. But omicron keeps its knuckles bent, hiding the bits that antibodies will attack, Acharya and colleagues reported in a preprint, which has not been peer reviewed yet, posted January 26 at bioRxiv.org.
Acquiring more mutations
The omicron variant of the coronavirus is more transmissible and can evade the immune system better than earlier versions of the virus, thanks in large part to mutations in spike protein. Shown here in red are locations of the mutations in the spike proteins of the delta (left) and omicron (right) variants.
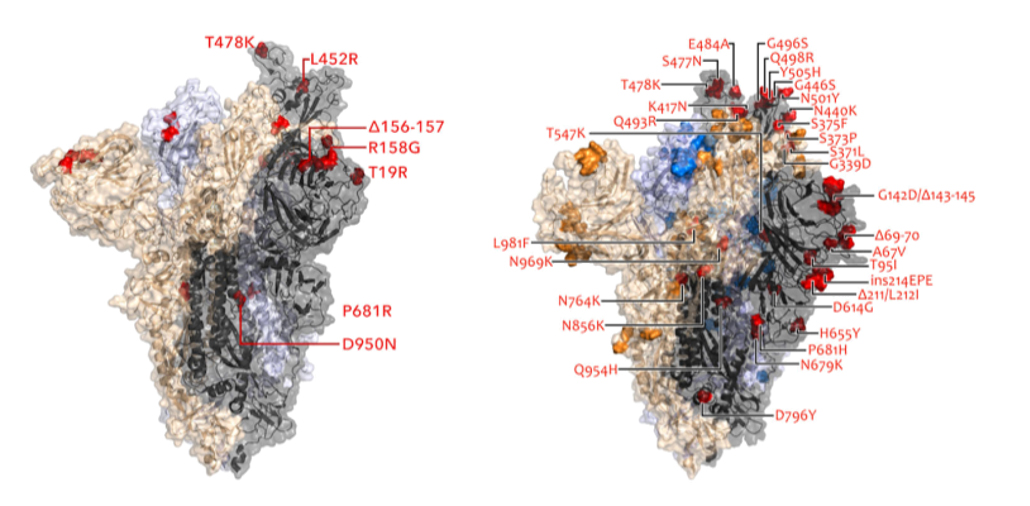 S. Kumar et al/Journal of Medical Virology 2021
S. Kumar et al/Journal of Medical Virology 2021
This closed-fist strategy helps the variant evade the immune system. But the fingers eventually have to extend in order to grasp ACE2. Some mutations essentially spring-load omicron’s claw so that it can shoot out at least one finger to snag ACE2. That ensures some of the spike proteins studding the surface of the virus are always pointing the way to infect cells, Acharya and colleagues found.
Delta and alpha variants of the coronavirus were also likely to have multiple extended fingers, but those variants’ efforts to open up “went way too far … to the point that they lost control and fell apart,” Acharya says. Omicron contains mutations that stabilize the spike protein through hydrogen bonds — weak electrostatic connections between a positively charged hydrogen atom in one molecule with a negatively charged atom in another molecule. As a result, omicron doesn’t become floppy the way the earlier variants do, the Duke group discovered. Another group also found new hydrogen bonds and other connections that help omicron keep itself together, researchers reported January 20 in Science. “I think omicron has struck just the right balance,” Acharya says.
2. With omicron, opposites really attract.
Simple attraction may be one key to omicron’s success.
“In the cellular environment, it can be difficult to find your partner,” says computational biologist Hin Hark Gan of New York University. Mutations in omicron’s receptor binding domain give that fingerlike portion of the protein a positive electric charge, Gan and NYU colleagues in New York City and Abu Dhabi reported February 14 at bioRxiv.org.
That electric charge complements ACE2’s negative charge, creating an electrostatic force that attracts the two proteins like a static-charged balloon to a wall, even over relatively long distances, the researchers propose in their preliminary report. Omicron’s electrostatic attraction to ACE2 is three to five times greater than more neutrally charged delta’s, the team found. The electrical attraction may make it easier for omicron to locate ACE2 on cells. Once the virus gets close to the human protein, other types of forces, including hydrogen bonds, cement the connection, he says.
Changing charge
Mutations in the omicron spike protein (right) have made it more positively charged (blue) than the delta variant’s spike (left). The charge attracts omicron toward the negatively charged ACE2 protein on human cells even across relatively large distances. Negative charges are shown in red and neutral parts of the protein in white.
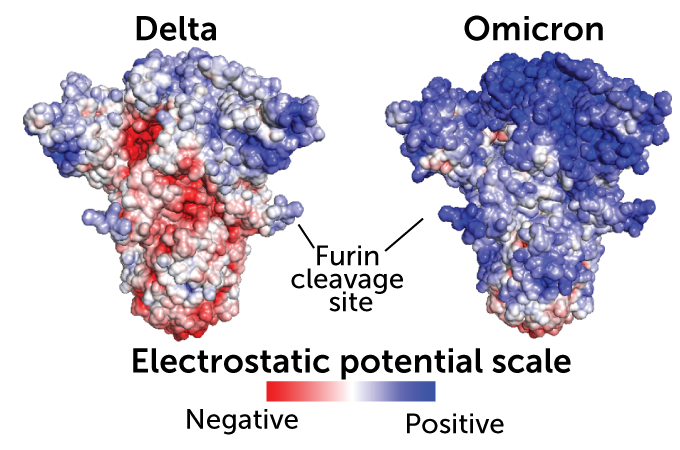 Image courtesy of Hin Hark Gan, NYUImage courtesy of Hin Hark Gan, NYU
Image courtesy of Hin Hark Gan, NYUImage courtesy of Hin Hark Gan, NYU
3. Omicron may sneak in a cell’s back door.
Multiple teams around the world have recently proposed another thing that makes omicron special: The variant may not use the same entry route into cells that earlier versions of the virus use.
There are two major ways the coronavirus can enter cells (SN: 8/2/20). Both start with grabbing ACE2. In the direct route, a scissorslike protein called TMPRSS2 snips away part of the spike protein, revealing a portion that allows the virus to fuse with human cells and immediately dump its RNA inside to make new viruses. That is the way all previous versions of the SARS-CoV-2 have entered human cells.
But omicron may take a back door through a compartment inside the cell membrane called an endosome. There, a different scissorslike protein called cathepsin L cleaves the spike protein to allow the virus to dump its payload into the cell.
Omicron doesn’t use the TMPRSS2 pathway efficiently and relies more on cathepsin L to get into cells, two groups of researchers independently reported February 1 in Nature. As a result, omicron doesn’t fuse as well with cell membranes as delta does, those teams and other preliminary reports suggest.
That could seem like a handicap. But for omicron, it may be a good thing, Liu of Ohio State says. While the virus needs to fuse with cell membranes to get inside and replicate, too much fusibility may lead cells to merge with each other and die, he says. That would leave the virus with nowhere to copy itself.
Omicron may have struck the perfect balance between being fusible enough to enter cells, but not enough to kill its host, he says. This characteristic may also make omicron less likely to cause severe disease.
A different door
Omicron may use an alternate entry route into cells compared with other coronavirus variants, some research suggests. After latching on to an ACE2 protein on a host cell’s surface, SARS-CoV-2 (red) usually goes through the front door, where a scissorslike protein called TMPRSS2 cuts off a part of the spike protein to unlock the door (as illustrated in the panel at left). But some data indicate omicron may use a back door, entering cells through endosomes, where a protein called cathepsin L is the locksmith (right).
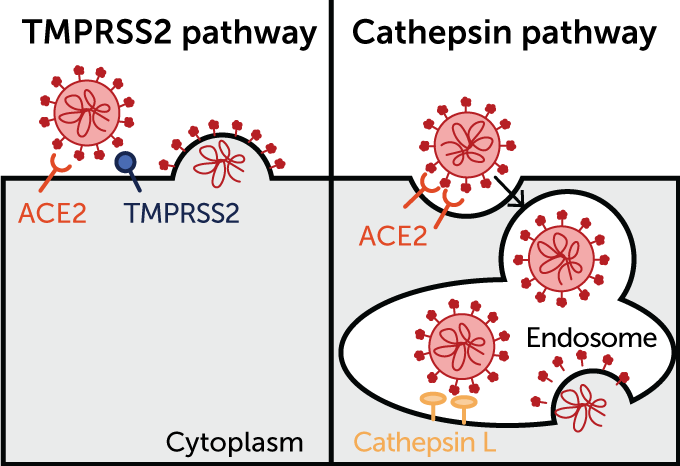 Tianling Ou et al/ bioRxiv.org 2020Tianling Ou et al/ bioRxiv.org 2020
Tianling Ou et al/ bioRxiv.org 2020Tianling Ou et al/ bioRxiv.org 2020
Once in the endosome, though, the viruses come up against a gang of protein guards called IFTIMs that block entry. But both delta and omicron have that problem licked: They breeze past those backdoor guards, researchers in the United Kingdom reported January 3 at bioRxiv.org.
Still, some researchers are not convinced that omicron uses the endosome back door into cells. In mimics of human airways grown in lab dishes, omicron used the direct route, but may be using a different — and still unknown — set of scissors than TMPRSS2, Bart Haagmans, a virologist at Erasmus Medical Center in Rotterdam, the Netherlands, and colleagues reported January 20 at bioRxiv.org.
Haagman’s team infected various types of cells with omicron, delta or earlier versions of the coronavirus. In the first couple of days of infection, omicron infected more cells than delta did, and replicated faster, giving omicron a competitive edge.
Omicron was able to infect cells that don’t have an endosome back door but do have TMPRSS2. It infects these cells much less efficiently than earlier versions of virus do. In another experiment, a chemical that blocks cutting by TMPRSS2 and other similar scissors proteins stopped omicron from breaking into cells, suggesting that some sort of spike cutter is still needed. Together, those results indicate that omicron uses TMPRSS2 inefficiently and might use different scissors all together, the researchers conclude. Those unknown scissors aren’t on lung cells, as omicron has a hard time breaking into those cells in lab dishes, the team found. That work is still preliminary, Haagmans stresses, and it is difficult to predict from studies of cells grown in lab dishes how the virus will behave in humans.
Acharya’s group sees another possibility: Omicron may not need scissors to trim it before it can enter cells. Some of omicron’s mutations may expose the fusion peptide portion of the spike responsible for melding the virus with cells. It’s possible that omicron could fuse directly with cells without being snipped, she speculates.
Omicron may use multiple routes for cell entry, depending on which type of cell it is infecting and what host proteins are available, Liu says. “It’s mixed. It’s not one or the other,” he says. Using different scissors or entry routes would allow omicron to break into potentially more types of cells than other versions of the virus could crack.
But the entry point may not matter as much as other of omicron’s characteristics, Liu says. “My take is that the major reason that this [version of the] virus spreads so fast is because it evades antibody protection.”
4. Omicron is evasive.
As omicron took over globally, it quickly became obvious that the variant could slip past antibodies and patrolling immune cells. Unvaccinated people remained the most likely to get infected, with 3,230.1 of every 100,000 unvaccinated people developing a case of COVID-19 at the height of the omicron peak on January 8, according to the U.S. Centers for Disease Control and Prevention.
But a startling 1,467.31 of every 100,000 fully vaccinated people also got breakthrough infections at that time. And fully vaccinated people who got a booster dose were still being infected at a rate of 1,053.6 for every 100,000 people on January 8. That far exceeds even slippery delta’s peak rate of 699.12 of every 100,000 unvaccinated and 140.8 of every 100,000 fully vaccinated people on August 28.
A study of the Moderna vaccine’s effectiveness conducted at Kaiser Permanente Southern California in December found that two doses of the mRNA vaccine were about 80 percent effective at protecting against delta infection from 14 to 90 days after inoculation. That effectiveness slipped to 68.9 percent for people three to six months out from their second shot. A booster shot raised the effectiveness against delta infection back to nearly 94 percent, though it dipped to 86 percent after two months. Both two and three doses of the vaccine gave better than 99 percent protection against hospitalization from delta, researchers reported February 21 in Nature Medicine.
Contrast those figures with omicron. Two doses of Moderna’s vaccine were 44 percent effective at preventing omicron infection from 14 to 90 days after getting the shot, quickly dipping to 23.5 effectiveness in the three- to six-month period after getting jabbed. A booster shot brought the effectiveness against omicron infection up to 71.6 percent for about two months, after which it declined to 47.4 percent. Against hospitalization from omicron, two doses were 84.5 percent effective. A booster dose brought effectiveness against hospitalization back above 99 percent.
See all our coverage of the coronavirus outbreak
How did omicron manage to get past the highly effective vaccine? The variant swapped out or is missing some of the spots on the spike protein where antibodies latch on to other variants, multiple studies show. And new data that hasn’t yet been vetted by peer-review may suggest other reasons omicron is so sneaky.
First, the variant’s spike protein has that closed-fist stance that protects its ACE2-grabbing bits from antibodies. It has also changed shape slightly to tuck away other spots where antibodies can bind, helping the protein escape from the immune system, Acharya and colleagues found. For instance, one popular hitching post for antibodies is called the N-terminal domain, but there are so many mutations that, “omicron has completely destroyed the N-terminal domain for antibodies. Nothing binds there,” she says.
Another evasion tactic: Omicron is especially good at spreading from cell to cell where the immune system can’t catch it as easily as when it is outside of cells, Liu and colleagues reported December 20 at bioRxiv.org. Despite not being good at fusing cells together, the variant is nearly 5 times better at cell-to-cell spread than an early version of the virus.
Plus, the same electric charge that helps omicron’s receptor binding domain to hold ACE2 may repel antibodies, the New York University researchers found. The team tested a handful of monoclonal antibodies taken from people who had recovered from COVID-19 early in the pandemic. Just like the omicron spike protein, those antibodies all carried positive electrical charges, which may act like a force field keeping antibodies away from the spike protein.
A strategically placed sugar molecule stuck to the receptor binding domain may also obscure the spike protein from immune system attack, researchers at the University of Wisconsin–Madison reported February 10 at bioRxiv.org.

Sign Up For the Latest from Science News
Headlines and summaries of the latest Science News articles, delivered to your inbox
Client key* E-mail Address* Go
Thank you for signing up!
There was a problem signing you up.
5. Even with many mutations, omicron seems relatively mild.
About the only good news about omicron is that it is less likely than the delta variant to cause severe disease. “It is definitely less virulent than delta,” says William Hanage, an epidemiologist at the Harvard T.H. Chan School of Public Health in Boston.
But there are challenges to pinpointing just how dangerous omicron may be, Hanage and Harvard colleague Roby Bhattacharyya discussed February 2 in the New England Journal of Medicine. Hospitals full of COVID-19 patients infected with omicron attest that the variant still has teeth and can cause severe illness or kill people. While vaccines and immunity from prior or breakthrough infections may blunt omicron’s edge, its increased infectiousness and slipperiness may offset its reduced nastiness.
Even so, “you would definitely rather have 750,000 daily cases of omicron than delta, because delta would be much worse,” Hanage says. For instance, the risk of dying from omicron is about 60 percent lower than the risk of death from a delta infection, the United Kingdom’s Health Security Agency reported February 11.
One reason for omicron’s relative mildness may be that it doesn’t replicate as well in lung cells as it does in the airways that feed the lungs, researchers in Hong Kong reported February 1 in Nature. That finding, initially reported in December, has also been confirmed with preliminary work from Haagmans and others (SN: 12/21/21).
Another omicron Achilles’ heel may be that it triggers interferon responses, the frontline of the immune system’s antiviral defenses, a study published January 21 in Cell Research found.
Gan of New York University speculates that omicron’s positive charge may cause the viral variant to get trapped in negatively charged mucus more easily than previous variants were.
Still, “omicron can be dangerous in a number of ways,” including landing vulnerable people in the hospital and sickening large numbers of hospital staff at the same time, Hanage says. “It is definitely milder, but that’s not a reason to chill.”
Add to that the fact that a sibling version of omicron called BA.2, which has some different mutations from the original omicron, has now burst on the scene (SN: 2/24/22). BA.2 seems to be slightly more infectious than the original version. Though cases of omicron are falling overall, BA.2’s proportion of those cases is growing, and some experts warn that it could prolong the omicron outbreak or lead to another wave of infections.

