A unique quantum effect in biology could be the key to understanding a common marker of Alzheimer’s, raising questions about current assumptions of the disease and informing the search for a cure.
Amyloid fibrils are fibrous protein structures in the brain that are associated with Alzheimer’s disease and dementia, among other neurodegenerative disorders. They are often the canonical target for experimental treatments for these diseases—usually in the form of drugs that seek to reduce the quantity of amyloids or prevent more from forming.
But many people who test positive for significant amounts of amyloid don’t develop dementia at all, and so far, treatment regimens that target amyloid have not been successful. Another known indicator of Alzheimer’s is the so-called allostatic load, a general term for the cumulative burden of chronic wear-and-tear on the body. The more oxidative stress, the higher the load and the higher risk of dementia.
Previously, a group of researchers found that a certain quantum effect—single-photon superradiance—could survive the turbulent environment of the human body in networks of the amino acid tryptophan, and could potentially mitigate oxidative stress in the body.
Now that group, led by Dr. Philip Kurian, principal investigator and founding director of the Quantum Biology Laboratory at Howard University in Washington, D.C., has established that these tryptophan networks have an even stronger ability to harness superradiant effects in amyloid fibrils than in the structures they studied previously.
The result, published in Frontiers in Physics, has prominent implications for the role of amyloid in Alzheimer’s disease.
“Our previous experimental confirmation of single-photon superradiance in protein fibers encouraged us to examine other neurobiological architectures, including amyloid fibrils,” said Kurian.
“While the superradiant enhancement of the quantum yield we saw previously was modest though detectable, our predicted superradiant enhancement for amyloid fibrils is enormous, up to five times the quantum yield of an individual tryptophan molecule. This finding has the potential to transform available treatments for dementia, and to revolutionize our understanding of information processing throughout the web of life.”
Oxidative stress, a contributing factor linked with Alzheimer’s, occurs when the body produces a large number of free radicals, which can emit damaging, high-energy UV photons. Single-photon superradiance is a quantum phenomenon where a collective network of molecules can very efficiently absorb these high-energy light particles and re-emit them at a lower, safer energy.
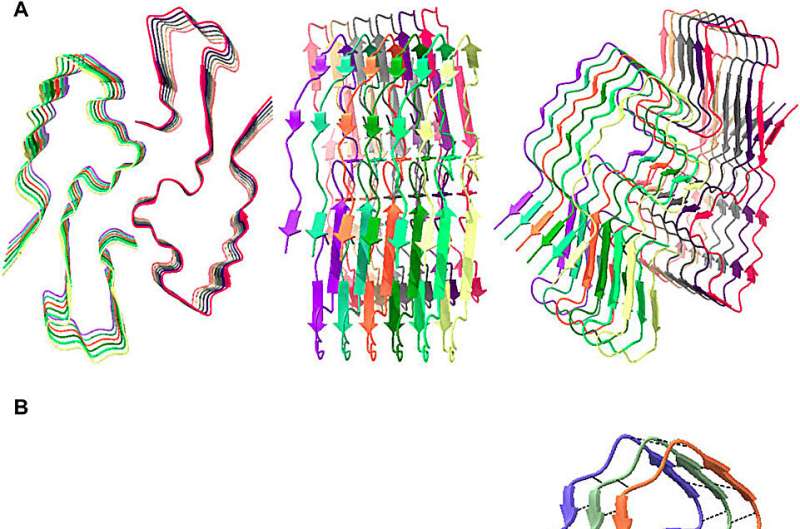
Because many amyloid fibrils have a very high density of tryptophans arranged in multiple helices, their ability to absorb damaging photons and downconvert the energy—photoprotection—is much stronger than anyone suspected before.
This could suggest that amyloid, rather than being a cause of Alzheimer’s, is actually the body’s adaptive response to a stressful environment that is awash with a higher proportion of UV photons from free radicals.
“The Kurian group has made an outstanding scientific contribution in elucidating the potential role of amyloid fibrils in mitigating oxidative stress and photophysical damage,” said Professor Lon Schneider, director of the USC California Alzheimer’s Disease Center, who was not involved in the research.
“This work has profound implications for understanding the pathophysiology of Alzheimer’s disease, as researchers generally work under the assumption that amyloid must be the proper target for treatment. On the contrary, Kurian’s work suggests that, rather than a cause of the disease, amyloid aggregation and fibril formation are a protective response.”
The next step is to validate this prediction experimentally, but Kurian also wants colleagues in biology and neuroscience to start thinking more broadly about how quantum perspectives are an essential part of the life sciences.
“We want to help others see that the interactions of light and quantum matter have significant relevance to all living systems,” he said.
The first author on the paper, Mr. Hamza Patwa, is a 2024 Barry Goldwater Scholar and a senior undergraduate intern in the Quantum Biology Laboratory.
“For me,” he said, “this work represents what true science is supposed to be. To make such a cognitive leap, one has to be versed in several different disciplines: open quantum systems, computational biology, and photophysics. It has taught me that science doesn’t always have to be separated into mutually exclusive categories.
“When we try to use tools from whichever subfields are necessary to solve a problem, this is where the awesome explanatory power of science is revealed.”
More information:
Hamza Patwa et al, Quantum-enhanced photoprotection in neuroprotein architectures emerges from collective light-matter interactions, Frontiers in Physics (2024). DOI: 10.3389/fphy.2024.1387271
Provided by
Howard University
Citation:
Quantum optical phenomenon in the brain challenges conventional view of amyloid in Alzheimer’s (2024, August 28)
retrieved 29 August 2024
from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.

