Cystic fibrosis (CF) is a life-threatening genetic disease affecting multiple organ systems, with pancreatic dysfunction representing a critical and often overlooked complication. A recent study published in eGastroenterology introduces young rabbits with CF as a novel and accessible model to study CF-related pancreatic endocrine pathology. This model offers an unprecedented opportunity to deepen our understanding of CF-related diabetes (CFRD), a condition affecting up to 50% of adults with CF.
CF is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene, resulting in abnormal chloride and sodium transport across epithelial cells. While advancements like Trikafta have significantly improved pulmonary outcomes, pancreatic complications—especially endocrine dysfunction—continue to challenge patients’ quality of life.
The present study, led by Dr. Jie Xu and colleagues, explores the spontaneous development of pancreatic lesions and glucose metabolism abnormalities in CF rabbits, offering new insights into the disease’s pathophysiology.
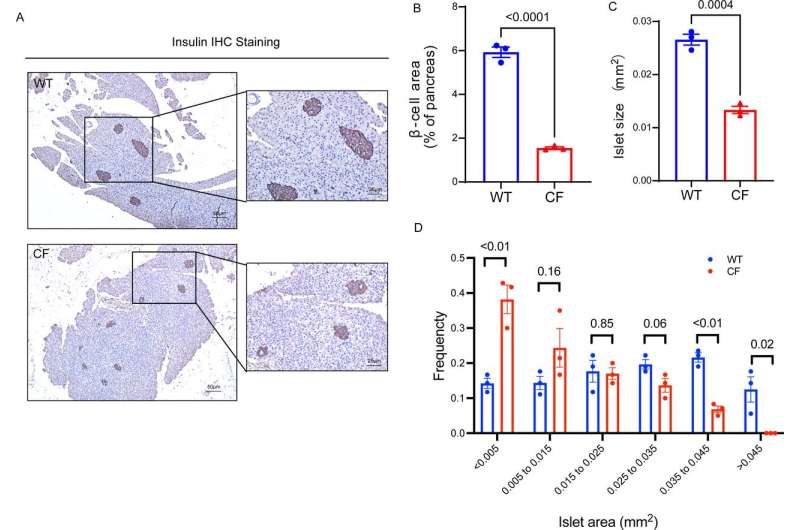
The team developed their CF rabbit model using CRISPR/Cas9 gene-editing technology. Rabbits with CF exhibited hallmark pancreatic changes, including fibrosis, vacuolar degeneration, and metaplasia of mucus-secreting epithelial cells. The size of insulin-producing pancreatic islets in these animals was significantly smaller than in wild-type controls, correlating with lower circulating insulin levels and compromised glucose metabolism.
“Our findings suggest that rabbits with CF replicate key aspects of CF pancreatic disease,” said Dr. Xu, corresponding author and a researcher at the University of Michigan Medical School. “This positions them as a valuable model for translational research on CFRD and related conditions.”
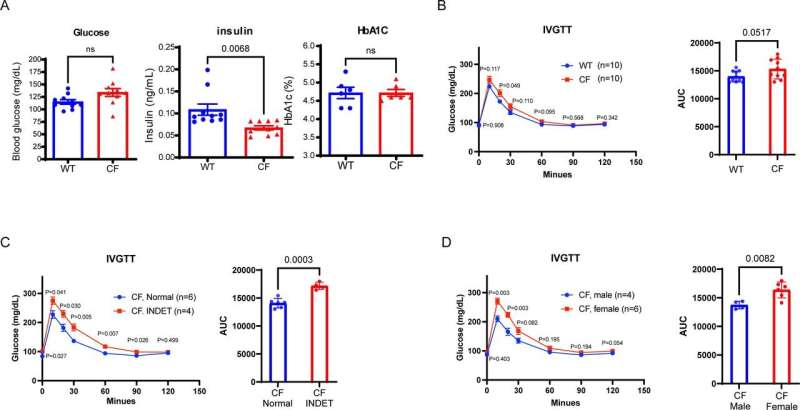
One of the study’s key outcomes was identifying an indeterminate glucose tolerance (INDET) stage in young CF rabbits, a precursor to CFRD observed in human patients. This stage featured delayed glucose clearance and reduced insulin secretion, paralleling early signs of diabetes progression in humans. Interestingly, female CF rabbits were more prone to INDET-like phenotypes, mirroring sex-based differences observed in human CFRD prevalence.
The implications of this work extend far beyond basic research. Rabbits with CF provide an alternative to existing large-animal models, such as pigs and ferrets, which are expensive and/or require specialized care. In contrast, rabbits are cost-effective, easier to handle, and widely used in laboratory settings, making them accessible to a broader range of researchers.
“As the CF community shifts its focus to age-related and metabolic complications, our rabbit model becomes increasingly relevant,” Dr. Xu explained. “With improved life expectancy due to CFTR modulators, understanding and mitigating CFRD will be critical for enhancing patients’ quality of life.”
The study also highlights the broader significance of CF-related pancreatic pathology. While exocrine dysfunction and pancreatic insufficiency are well-documented in CF, endocrine dysfunction—manifested as CFRD—presents unique challenges. CFRD combines features of both type 1 and type 2 diabetes, with insulin deficiency and resistance contributing to its progression. Current treatments focus primarily on symptom management, underscoring the urgent need for innovative therapeutic strategies.
By establishing rabbits as a viable model for CFRD research, the authors aim to facilitate the development of targeted interventions. Early-stage therapies, such as those addressing the INDET phase, could significantly delay or prevent the onset of full-blown diabetes in CF patients. Furthermore, this model allows for evaluating emerging treatments, including those designed to modulate pancreatic inflammation, fibrosis, and insulin production.
Future directions of the research include exploring the long-term progression of pancreatic disease in CF rabbits and evaluating the impact of CFTR modulators like Trikafta on endocrine outcomes.
In conclusion, this work represents a significant step forward in CF research. By leveraging the CF rabbit model’s unique advantages, scientists can better understand the complex interplay between CFTR dysfunction and pancreatic disease. This knowledge promises to improve outcomes for thousands of patients living with CF and its complications.
More information:
Xiubin Liang et al, Endocrine pathology in young rabbits with cystic fibrosis, eGastroenterology (2024). DOI: 10.1136/egastro-2024-100102
Provided by
First Hospital of Jilin University
Citation:
New animal model offers insights into pancreatic disease and diabetes (2025, January 17)
retrieved 17 January 2025
from https://medicalxpress.com/news/2025-01-animal-insights-pancreatic-disease-diabetes.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.

