Shimmering, gelatinous comb jellies wouldn’t appear to have much to hide. But their mostly see-through bodies cloak a nervous system unlike that of any other known animal, researchers report in the April 21 Science.
In the nervous systems of everything from anemones to aardvarks, electrical impulses pass between nerve cells, allowing for signals to move from one cell to the next. But the ctenophores’ cobweb of neurons, called a nerve net, is missing these distinct connection spots, or synapses. Instead, the nerve net is fused together, with long, stringy neurons sharing a cell membrane, a new 3-D map of its structure shows.
While the nerve net has been described before, no one had generated a high-resolution, detailed picture of it.
It’s possible the bizarre tissue represents a second, independent evolutionary origin of a nervous system, say Pawel Burkhardt, a comparative neurobiologist at the University of Bergen in Norway, and colleagues.
Superficially similar to jellyfish, ctenophores are often called comb jellies because they swim using rows of beating, hairlike combs. The enigmatic phylum is considered one of the earliest to branch off the animal tree of life. So ctenophores’ possession of a simple nervous system has been of particular interest to scientists interested in how such systems evolved.
Previous genetics research had hinted at the strangeness of the ctenophore nervous system. For instance, a 2018 study couldn’t find a cell type in ctenophores with a genetic signature that corresponded to recognizable neurons, Burkhardt says.
Burkhardt, along with neurobiologist Maike Kittelmann of Oxford Brookes University in England and colleagues, examined young sea walnuts (Mnemiopsis leidyi) using electron microscopes, compiling many images to reconstruct the entire net structure. Their 3-D map of a 1-day-old sea walnut revealed the funky synapse-free fusion between the five sprawling neurons that made up the tiny ctenophore’s net.
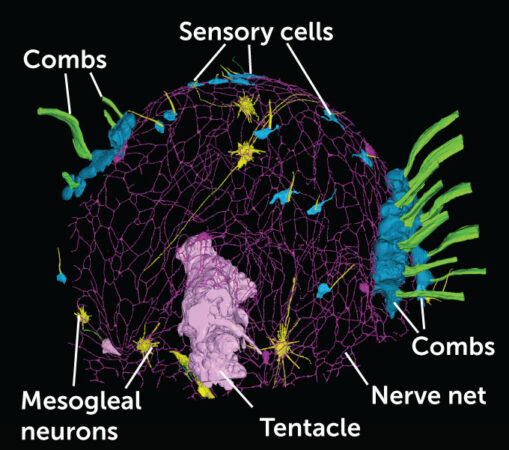 This 3-D reconstruction of the sea walnut’s nervous system reveals the structure of its nerve net (purple) as well as nearby structures and other nervous system components. Two kinds of nerve cells — sensory cells (light blue) and mesogleal neurons (yellow) exist outside the nerve net. How they interact with the nerve net is still unknown, as is the role of the mesogleal neurons. Those nerve cells exist in the animal’s gelatinous body layer and may provide nutrients or other support to the nerve net.Pawel Burkhardt and Maike KittelmannThis 3-D reconstruction of the sea walnut’s nervous system reveals the structure of its nerve net (purple) as well as nearby structures and other nervous system components. Two kinds of nerve cells — sensory cells (light blue) and mesogleal neurons (yellow) exist outside the nerve net. How they interact with the nerve net is still unknown, as is the role of the mesogleal neurons. Those nerve cells exist in the animal’s gelatinous body layer and may provide nutrients or other support to the nerve net.Pawel Burkhardt and Maike Kittelmann
This 3-D reconstruction of the sea walnut’s nervous system reveals the structure of its nerve net (purple) as well as nearby structures and other nervous system components. Two kinds of nerve cells — sensory cells (light blue) and mesogleal neurons (yellow) exist outside the nerve net. How they interact with the nerve net is still unknown, as is the role of the mesogleal neurons. Those nerve cells exist in the animal’s gelatinous body layer and may provide nutrients or other support to the nerve net.Pawel Burkhardt and Maike KittelmannThis 3-D reconstruction of the sea walnut’s nervous system reveals the structure of its nerve net (purple) as well as nearby structures and other nervous system components. Two kinds of nerve cells — sensory cells (light blue) and mesogleal neurons (yellow) exist outside the nerve net. How they interact with the nerve net is still unknown, as is the role of the mesogleal neurons. Those nerve cells exist in the animal’s gelatinous body layer and may provide nutrients or other support to the nerve net.Pawel Burkhardt and Maike KittelmannThe conventional view is that neurons and the rest of the nervous system evolved once in animal evolutionary history. But given this “unique architecture” and ctenophores’ ancient position in the animal kingdom, it raises the possibility that nerve cells actually evolved twice, Burkhardt says. “I think that’s exciting.”
But he adds that further work — especially on the development of these neurons — is needed to help verify their evolutionary origin.
The origins of the animal nervous system is a murky area of research. Sponges — the traditional competitors for the title of most ancient animal — don’t have a nervous system, or muscles or fundamental vision proteins called opsins, for that matter. But there’s been mounting evidence to suggest that ctenophores are actually the most ancient animal group, older even than sponges (SN: 12/12/13).
If ctenophores arose first, it “implies that either sponges have lost a massive number of features, or that the ctenophores effectively evolved them all independently,” says Graham Budd, a paleobiologist at Uppsala University in Sweden who was not involved in the research.
If sponges emerged first, it’s still possible that ctenophores evolved their nerve net independently rather than inheriting it from a neuron-bearing ancestor, Burkhardt says. Ctenophores have other neurons outside the nerve net, such as mesogleal neurons embedded in a ctenophore’s gelatinous body layer and sensory cells, the latter of which may communicate with the nerve net to adjust the beating of the combs. So, it’s possible they’re a mosaic of two nervous systems of differing evolutionary origins.
But Joseph Ryan, a bioinformatician at the University of Florida in Gainesville, doesn’t think the results necessarily point to the parallel evolution of a nervous system. Given how long ctenophores have been around — especially if they are older than sponges — the ancestral nervous system may have had plenty of time to evolve into something weird and highly-specialized, says Ryan, who was not part of the study. “We’re dealing with close to a billion years of evolution. We’re going to expect strange things to happen.”
The findings are “one more bit of the jigsaw puzzle,” Budd says. “There’s a whole bunch we don’t know about these rather common and rather well-known animals.”
For instance, it’s unclear how the nerve net works. Our neurons use rapid changes in voltage across their cell membranes to send signals, but the nerve net might work quite differently, Burkhardt says.
There are reports of potentially similar systems in other animals, such as by-the-wind-sailor jellies (Velella velella). Studying them in detail, along with nerve nets in other ctenophore species, could determine just how unusual this synapse-less nervous system is.

