In a large laboratory cage, a male mosquito carries a genetic weapon that could launch the destruction of his species. That loss could also mean the end of the parasite that causes malaria.
The weapon, a self-replicating bit of DNA known as a gene drive, is one of the most anticipated and controversial tools being developed to stop mosquitoes from spreading diseases like malaria to humans.
The gene drive interferes with the insects’ ability to reproduce. It wiped out captive populations of mosquitoes in eight to 12 generations (SN: 10/27/18, p. 6) in a small lab study. In 2021, the technology worked in the large cages in Terni, Italy, too. Within as little as five to 10 years, this gene drive could be ready to test in the wild.
The first experimental release could be rolled out in Burkina Faso, Mali, Ghana or Uganda. In those locations, researchers are working with a nonprofit research consortium called Target Malaria to develop the gene drive carriers along with other genetically engineered mosquitoes to fight malaria.
This research is driven by the idea that every tool available must be used to fight malaria, which sickened close to 241 million people in 2020 and killed 670,000 worldwide, mostly in Africa. Children 5 years old and younger accounted for about 80 percent of the continent’s malaria deaths, the World Health Organization says.
Because of malaria’s huge toll, large investments have been made to fight the disease, yielding preventive drugs, insecticide-treated bed nets and even malaria vaccines — one was recently recommended for use in sub-Saharan Africa (SN: 12/18/21 & 1/1/22, p. 32). These efforts are helping. But mosquitoes are developing resistance to insecticides, and some anti-malaria drugs may no longer work well.
“To go toward zero [cases], we need to have something that is transformational,” says Fredros Okumu, a mosquito biologist and director of science at Ifakara Health Institute in Tanzania.

Sign Up For the Latest from Science News
Headlines and summaries of the latest Science News articles, delivered to your inbox
Client key* E-mail Address* Go
Thank you for signing up!
There was a problem signing you up.
Gene drives might be the transformational answer people are looking for. Researchers are still refining and testing the technology, which was first devised in 2015 (SN: 12/12/15, p. 16). Though other types of genetically altered mosquitoes have been released in Brazil, the United States and elsewhere, those altered genes spread slowly among wild populations (SN Online: 3/9/22). Gene drives could potentially spread to nearly ever member of a species quickly, forever altering the species or wiping it out.
But whether gene drives ever play a role in combating malaria may depend as much on social considerations as on science.
“A technology doesn’t work by technical strength alone. It works because it embeds into a social context,” says Ramya Rajagopalan, a social scientist at the University of California, San Diego. In the past, scientists “developed a technology in the lab, got it all set up and ready to go, and then you go to the stakeholders and say, ‘Hey, we have this great technology, do you want to use it?’ ”
If people reject that sort of offer, as has happened with some genetically modified crops, researchers often think, “If [the public] only knew enough about the technology, they’d be more accepting,” Rajagopalan says. But more often the failure comes because the researchers “don’t include community voices from the outset in the design and the implementation.”
Because of the possibility of forever altering ecosystems, the European Union has already said “no” to using gene drives there. But Africa is where a gene drive might one day help defeat malaria. Researchers are hoping to eventually release gene drives on the continent, but must first get public consensus. To that end, scientists are looking for ways to involve members of the public in research, and learn about local priorities and how to talk about the technology.
 In Uganda, Target Malaria staff member Victor Balyesima collects mosquitoes for experiments. Local residents often help with this effort.Target Malaria
In Uganda, Target Malaria staff member Victor Balyesima collects mosquitoes for experiments. Local residents often help with this effort.Target Malaria
Rattling the cage
No one is ready to let mosquitoes carrying gene drives out of the lab yet. For now, researchers are doing tests with mosquitoes in captivity to get an idea of whether the technology will work as planned. In the Terni cage trials, scientists used small rooms, setting humidity levels, lighting and other characteristics to mimic some of the conditions the mosquitoes might encounter in the wild.
In cages almost 5 cubic meters big — about the size of a small dressing room — containing hundreds of Anopheles gambiae mosquitoes, scientists added male members of the same species that carried the engineered change to their DNA.
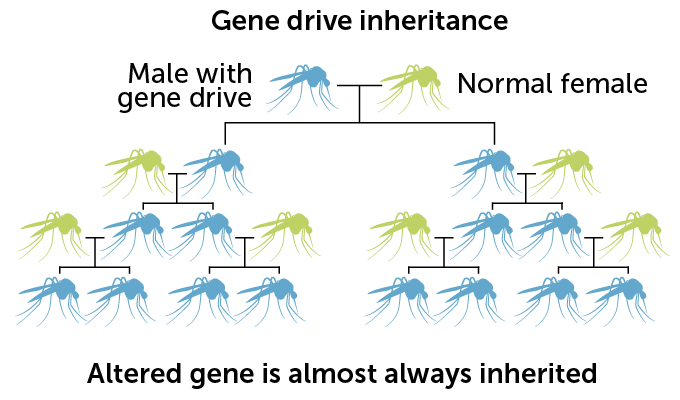
The gene drive used for this experiment is built on the molecular scissors known as CRISPR/Cas9. Male mosquitoes are engineered to carry the gene drive, which consists of instructions for making the DNA-cutting enzyme Cas9 and an RNA that guides the enzyme to the gene to be cut. When an engineered male mates with an unaltered female, Cas9 snips a gene called doublesex inside the fertilized egg. As the egg tries to repair the cut, the gene drive from the father’s doublesex gene is pasted over the copy of the gene inherited from the mother. So the offspring gets two copies of the gene drive, instead of one.
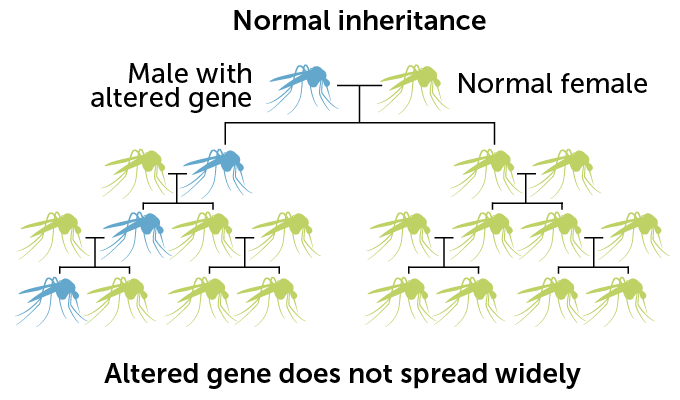
Normally, any particular version of a gene has a 50 percent chance of being passed from parent to offspring. But with the copy-and-paste CRISPR system, gene drive–carrying mosquitoes pass the drive to about 96 percent of male progeny and more than 99 percent of females. With that genetic cheat, the gene drive spreads rapidly through the population.
Upping the odds
With other forms of genetic engineering, the altered gene follows normal rules of inheritance and is passed on to only 50 percent of offspring. Gene drives can paste themselves into a gene inherited from an unaltered parent, ensuring the genetic change gets passed on more often.
 E. Otwell, M. Telfer
E. Otwell, M. Telfer
The doublesex gene is essential for the development of female mosquitoes. When the gene doesn’t work, “the mosquito itself doesn’t work,” says Ruth Müller, chief ecologist and entomologist at the Institute of Tropical Medicine in Antwerp, Belgium. The gene drive breaks the gene.
Female offspring that inherit two copies of a broken doublesex gene develop mouthparts and genitalia that are closer to the male form. Those females are sterile, and they cannot bite people with their malformed mouthparts. Unable to bite, those mosquitoes can’t transmit malaria-causing parasites from their bodies to humans.
In those naturelike cages in Terni, when gene drive–carrying mosquitoes were introduced, the populations died out in 245 to 311 days, researchers reported in July 2021 in Nature Communications. In two cages where no gene drive mosquitoes were added, mosquito populations lived normally to the end of the experiment.
This was the first proof that the gene drive might work under almost real-world conditions, says Müller, one of the study’s leaders. But there is still a lot to learn about drives, she says, including how they will affect mosquito populations in the wild, whether they can slow malaria’s spread and importantly, what the impact will be on other creatures in the environment.
Getting those answers will determine the feasibility of moving forward scientifically. They will also play a big role in whether the public agrees to releasing a tool that could intentionally drive a species toward extinction.
Population crash
In large cages with about 570 mosquitoes, researchers added about 70 gene drive–carrying males. As the gene drive spread, more females became sterile and egg production crashed (red and blue lines). The gene drives worked faster than computer simulations suggested (gray lines). In control cages with no gene drive, egg production continued as usual (horizontal dotted black line).
Mosquito egg output differs with and without gene drive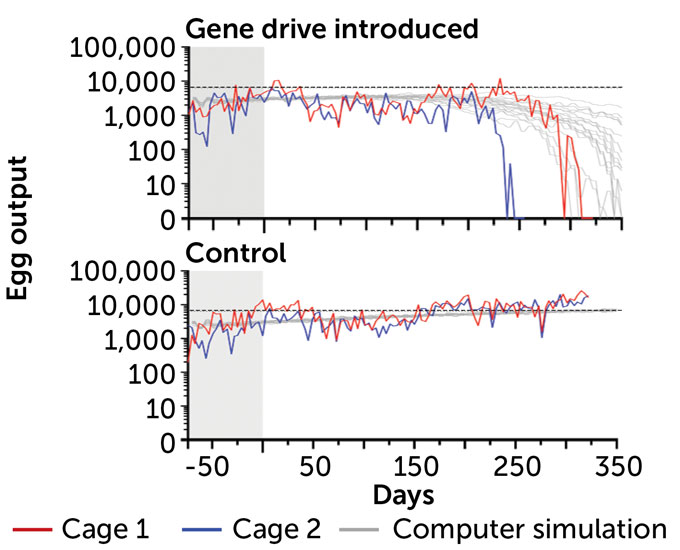 A. Hammond et al/Nature Communications 2021A. Hammond et al/Nature Communications 2021
A. Hammond et al/Nature Communications 2021A. Hammond et al/Nature Communications 2021
Considering all possibilities
While Müller’s and other Target Malaria science teams based in Africa, Europe and North America refine gene drives, other affiliated and independent groups are mapping out what releasing a gene drive could do to the planet. “Right now there are a lot of theoretical discussions,” Müller says. It’s important to gather data to “fill the debate with more facts” about the real risks and benefits, she says.
At least 46 theoretical harms could arise from the use of gene drives on mosquitoes, researchers reported in March 2021 in Malaria Journal. Those potential downsides include reductions in pollinators and other species directly or indirectly related to the disappearance of the mosquitoes. It’s possible that people could develop allergic reactions to the bite of mosquitoes carrying a single copy of the gene drive, or to fish that eat the altered mosquito larvae. There could be a decline in water quality caused by large numbers of mosquito larvae dying. There’s even a set of scenarios in which malaria cases increase if, for instance, mosquito species that are better malaria spreaders take over in areas where a gene drive has thinned out less-troublesome mosquitoes.
Dreaming up possible nightmare consequences was an exercise intended to tell researchers what they might need to plan for and test before releasing gene drive mosquitoes into the wild. At workshops held in 2016 through 2019 in Ghana, Kenya, Botswana, Gabon and the United States, researchers worked out a chain of events that might lead to each of those potential harms.
The list of 46 possibilities focused on four areas that African leaders said were most important to protect: biodiversity, human and animal health, and water quality. By identifying these hypothetical hazards, researchers can begin calculating the likelihood of a harm happening and how bad it could be, says report coauthor John Connolly, a senior regulatory scientist for Target Malaria who is based at Imperial College London.
“You probably never really finish a risk assessment, but you get a clearer understanding of the risks and uncertainties,” Connolly says. Target Malaria and independent groups hope to answer some questions by examining data collected from the release of genetically altered mosquitoes that don’t carry gene drives.
Studies of biological pest control mechanisms — such as releasing a predator to eradicate an invasive species (remember invasive cane toads in Australia [SN Online: 10/14/14]) — may also provide some clues about how gene drives may spread, says Keith Hayes, who leads a risk assessment team at the Commonwealth Science and Industrial Research Organization’s Data61 in Hobart, Australia.
Some questions may never truly be answered unless gene drives are released. Scientists can experiment and simulate what might happen, but “at some point you have to say, ‘We don’t know everything. We can’t know everything. There may be surprises,’ ” Hayes says. That’s when a decision will need to be made about a release based on what is known about the risks and benefits.
High stakes
Even if those evaluations reveal downsides to gene drives, the potential benefits for human health and economics may far outweigh the risks, Müller argues.
“If you have a high burden of malaria, that costs a lot,” Müller says. “Children cannot go to school. People cannot go to work. That should also be considered if you talk about costs.”
Opponents of gene drives say it’s unfair to paint rejection of the unproven, potentially dangerous, technology as dooming children to death from malaria. “We are already not saving those children with measures [that would help] such as improving sanitation and the medical system,” says Mareike Imken, the European coordinator of the Stop Gene Drives campaign. Her organization is calling for a global moratorium on the release of gene drives until there is worldwide consensus on whether they are safe and necessary and how they should be regulated.
“We need the highest possible obstacle to using this high-risk … technology,” Imken says. Allowing gene drives to be tried against malaria would essentially unleash them for use against a wide variety of organisms, with potentially devastating ecological consequences, she says. Instead, the world should invest more in already proven methods of controlling and eradicating malaria.
But there are potential upsides to gene drives that current approaches, such as insecticides, don’t offer. “The stuff we have been doing for years has been intentionally designed to eradicate mosquitoes. It just didn’t do it. We’ve been spraying the hell out of them for years, and in the process killing a lot of other nontarget organisms,” Okumu says.
By replacing insecticides, gene drives might help save insects including bees, butterflies and other pollinators. And gene drives are designed to eliminate only the mosquito species that are dangerous, Okumu says. “Of all the 3,500 species … we need to target one, two, at maximum three of them.”
He’s referring to the handful of species in the Anopheles genus that are mostly responsible for spreading malaria. In Africa, the primary disease carriers are Anopheles gambiae and the look-alikes An. arabiensis, An. coluzzii and An. funestus.
While eradicating malaria is the goal, making mosquitoes extinct is mostly hyperbole, says Tony Nolan, a molecular biologist at the Liverpool School of Tropical Medicine in England.
“Extinction is not a likely outcome, nor even a desirable one. It’s not necessary to make the mosquito extinct to eliminate malaria,” says Nolan, one of the Target Malaria researchers developing gene drives. Geographic isolation may enable the gene drive to eliminate a local population of mosquitoes but nothing further afield. Mutations can arise in the Cas9 or guide RNA, causing the drive to stop working. Or other things might limit its spread.
But what would happen to the environment if a major mosquito species suddenly disappeared? Some researchers are trying to measure the ecological contributions of An. gambiae, including whether males pollinate plants visited for nectar. As of now, the mosquitoes’ biggest known value is as food for predators. Birds, fish and other animals that eat mosquitoes or their larvae usually aren’t picky about which species is for dinner. Only one species of spider is known to prefer Anopheles mosquitoes over other kinds.
Okumu’s experience leads him to think the malaria carriers wouldn’t be missed much. In some parts of eastern Africa, including Okumu’s home village in Tanzania, a combination of factors including prolonged dry seasons and insecticide and bed net use pushed An. gambiae out. “We have not seen — maybe because we didn’t measure [well enough] — any ecological challenges associated with the disappearance of Anopheles gambiae,” he says.
The mix of malaria carriers can vary considerably depending on local conditions. In Burkina Faso in western Africa, for instance, two villages had different mosquito populations: In Bana, to the northwest of the city Bobo-Dioulasso, about 90 percent of mosquitoes were An. coluzzii with An. gambiae making up 9 percent of the catch, researchers reported in 2019 in Malaria Journal. But on the southeastern side of the city, in the village of Pala, An. gambiae dominated, making up about 84 percent of mosquitoes caught. An. arabiensis accounted for about 10 percent, and An. coluzzii was about 6 percent of the catch in Pala.
If An. gambiae disappeared, one of the other species would fill the vacuum, Okumu says. That could be a good thing if the replacements don’t bite people as much or are lousy at spreading malaria. It could also be worse if the balance shifts toward a more voracious people-biter that easily spreads the parasites.
Malaria carriers
Though separated by only about 30 kilometers, the villages of Bana and Pala in western Africa’s Burkina Faso each have a different mix of malaria-carrying Anopheles species.
Mosquito mixes in two villages in Burkina Faso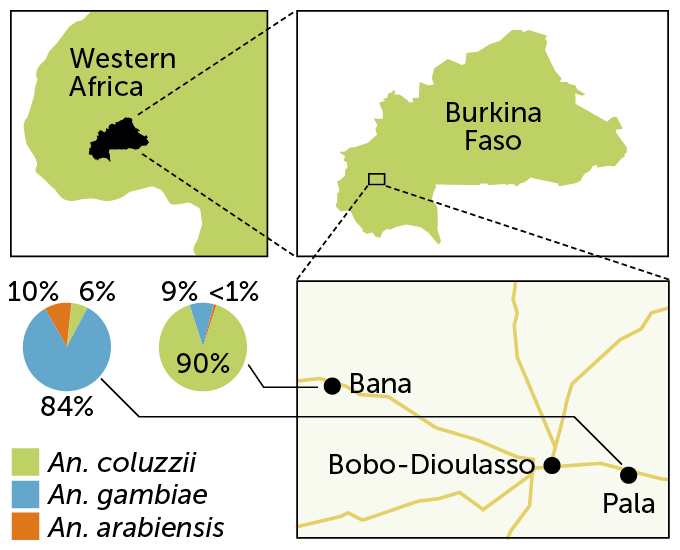 C. ChangC. Chang Source: P.S. Epopa et al/Malaria Journal 2019
C. ChangC. Chang Source: P.S. Epopa et al/Malaria Journal 2019
Community input
Beyond the scientific hurdles, researchers must also get the public on board with releasing the technology. Without public support, even a gene drive that works perfectly could be a no-go.
Not everyone agrees on when and how to get input. Okumu worries that asking the public whether they want gene drives before scientists have answers to some of the most pressing questions could backfire. “I would rather we know the true benefits, the true risks and gain a consensus around it, and then start engaging the communities,” he says.
Waiting until all the answers are in hand is a flawed approach, says Lea Pare Toe, a social scientist at the Institut de Recherche en Sciences de la Santé in Bobo-Dioulasso. “We should listen to [the community] and develop the science together,” says Toe, who works with Target Malaria to engage local people in the research.
At Bana, researchers didn’t start out talking about gene drives, or even genetic modifications, Toe says. First, the team had to clarify the connection between mosquitoes and malaria. They also had to dispel myths, such as eating fatty foods or sweet fruit can cause the disease. After an intensive engagement campaign from 2014 through 2019, researchers found that such false statements were far less accepted, the researchers reported in October 2021 in Malaria Journal.
Fact vs. fiction
Over five years, discussions with residents of Bana, Burkina Faso, cleared up some misconceptions about how malaria is transmitted. The talks were part of a Target Malaria effort to involve the community in plans to use genetically modified mosquitoes.
Community agreement on false statements about mosquitoes and malaria in 2014 and 2019
| Misconceptions about mosquitoes and malaria |
2014 % agree (Respondents: 179) |
2019 % agree (Respondents: 149) |
|---|---|---|
| All mosquitoes transmit malaria | 76.3 | 16.1 |
| Male mosquitoes transmit malaria | 77.7 | 10.1 |
| Seasonal fruits transmit malaria | 13.9 | 0.9 |
| Fatty foods transmit malaria | 34.6 | 1.3 |
Source: L.P. Toe et al/Malaria Journal 2021
Once people are clear on the causes of malaria, Toe and colleagues introduce the idea of genetics, and how researchers want to alter mosquitoes to combat malaria. People are generally OK with the uncertainty of research, she says. But they want to know more.
Residents pose specific questions about mosquito biology and ask how researchers can possibly work with such small creatures. They often ask whether the genetic alterations that make the mosquitoes sterile will transfer to humans. People “love the details,” Toe says.
Sometimes, creative approaches are needed to get concepts across. For instance, Target Malaria planned a first stage — releasing genetically sterilized male mosquitoes that won’t diminish mosquito populations — to help researchers collect data on how genetically altered mosquitoes stack up to normal ones in the wild.
Before those altered mosquitoes were set free, the organization wanted to ensure that Bana residents had a deep understanding of the project. Local leaders suggested a play. The scientists wrote a script, but the actors, a local storyteller and other community members revised it to improve storytelling. This helped forge an emotional connection with the audience, Toe and colleagues reported April 5 in Humanities and Social Sciences Communications.
 Community leaders in Bana, Burkina Faso, suggested that researchers use theater to explain important concepts about genetically sterilized mosquitoes. Actors (shown during a performance) and community members helped develop the script.Target Malaria
Community leaders in Bana, Burkina Faso, suggested that researchers use theater to explain important concepts about genetically sterilized mosquitoes. Actors (shown during a performance) and community members helped develop the script.Target Malaria
Meanwhile in Tanzania, although reluctant to move too soon with the public, Okumu and colleagues talked with community leaders and surveyed residents of 10 villages in the southeastern part of the country, where very few people had heard about genetically modifying mosquitoes. The aim of this 2019 effort was to understand community perceptions, rather than ask permission. People were intrigued by the idea of gene drives, but they had concerns about whether the mosquitoes would look and behave differently from local mosquitoes, the team reported in March 2021 in Malaria Journal.
Community members were also skeptical that targeting just one type of mosquito would be enough to reduce malaria transmission or decrease mosquito bites enough to keep communities on board with the project. It would be better, they said, to get rid of all the biting mosquitoes.
In a separate study done in 2019, people in Uganda who were already familiar with gene drives expressed similar concerns. But those participants anticipated problems if the mosquitoes cross national borders into a country opposed to the release, researchers reported in March 2021 in Malaria Journal. Researchers may have to seek permission to release gene drive mosquitoes on a multinational scale, instead of just getting local and national consent.
Gene drives may win hearts and minds because they will first be tried against disease-carrying mosquitoes “that are very, very much not beloved or charismatic or anything,” says developmental geneticist Kimberly Cooper of UC San Diego. “Do you know anyone who has sympathies for the mosquito? It’s probably the most hated animal on the planet.
“But there will always be people who are very concerned about genetically modified organisms and their release into the environment,” even if those organisms are mosquitoes, says Cooper, who is not involved with the malaria gene drive research but is developing a gene drive to use as a research tool in mice (SN Online: 1/23/19).
Still, the attraction of stamping out malaria is powerful. The benefits could be enormous. But whether they outweigh any environmental risks from the technology and whether the public will buy in to this radical approach remains to be seen.
“There are tons of unknowns,” Okumu says. “The question is, should we pursue it? If you ask me, it would be unethical not to.”
Vampire spiders might miss Anopheles mosquitoes … for a minute
If gene drives wiped out swaths of malaria-carrying mosquitoes, perhaps the only creature that would mourn the loss is a species of jumping spider found in the Lake Victoria region of Kenya and Uganda.
The spiders, Evarcha culicivora, share the mosquitoes’ taste for human and animal blood. “This vampire spider is perhaps the only species we know is heavily dependent on these [mosquitoes],” says Fredros Okumu, a mosquito biologist and director of science at Ifakara Health Institute in Tanzania. He’s referring to Anopheles mosquitoes, a main malaria spreader in Africa.
Blood is a unisex perfume for the spiders. Adults that have recently feasted on blood are more attractive to the opposite sex. Blood also provides nourishment for adult and baby spiders.
But the spiders’ mouthparts are unable to pierce skin or hides, says Fiona Cross, an arachnologist at the University of Canterbury in Christchurch, New Zealand. Instead, the spiders wait for mosquitoes to siphon blood from a person or animal, then the spiders pounce on the flying blood bags . “We call them mosquito terminators,” she says.
 Vampire spiders feed on human and animal blood, but rely on blood-filled Anopheles mosquitoes for their meals. If those insects disappear, they’ll be able to adapt.F. Cross
Vampire spiders feed on human and animal blood, but rely on blood-filled Anopheles mosquitoes for their meals. If those insects disappear, they’ll be able to adapt.F. Cross
While any blood-laden mosquito will do, Anopheles mosquitoes are Evarcha’s favorite, partly because of the mosquito species’ bottoms-up resting pose. While other mosquitoes rest with their abdomens parallel to a surface, Anopheles lift their rears into the air, especially helpful for baby spiders, which are able to creep under the tilted abdomen.
Baby spiders, which “basically resemble dots with eight legs,” scuttle under the mosquito, “leap up, grab the mosquito from underneath, and as the mosquito flies away, the little spiderlings hang on with their little fangs and have just enough venom to bring the mosquito down,” Cross says. “They have the feast of a lifetime.”
But that doesn’t mean gene drives would doom the spiders by eliminating large swaths of the mosquitoes, Cross says. “If Anopheles were wiped from the planet, I would say that the spiders could adapt.”

