Half a century ago, the first genetically modified organism ushered in a new era of biological innovation. To mark this anniversary, here are eight milestone GMOs. Many have had, or are poised to have, a dramatic impact on our lives.
1. Escherichia coli
In November 1973, geneticist Stanley Cohen and colleagues reported that they had built a plasmid, a ring of DNA, that carried a gene from another organism into an E. coli cell — the birth of genetic engineering (SN: 6/1/74). The team later showed that such modified cells could produce the protein associated with a foreign gene. E. coli has since been modified to mass-produce therapeutic drugs, break down plastics and more. “The most important GMO is the microbes that are used to make insulin,” says geneticist Matthew Cobb of the University of Manchester in England. In 1978, facing problems with insulin derived from pigs and cows, scientists engineered E. coli to make human insulin for treating diabetes (SN: 9/16/78). The lifesaving drug hit the market in 1982 (SN: 10/9/82).
2. Transgenic mice
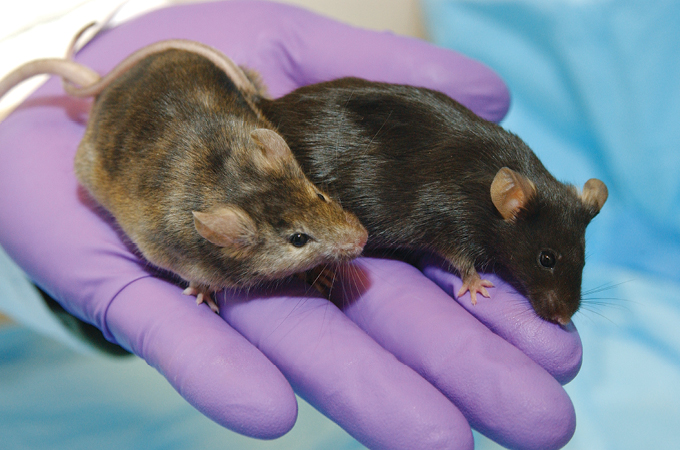
Mouse models are a go-to for scientists who want to study human disease in a controlled way in the lab. In 1974, biologists Rudolf Jaenisch and Beatrice Mintz laid the groundwork for these models by injecting DNA from simian virus into mouse embryos, which were later born with viral DNA in their genomes. In papers published in 1980 and 1981, a team led by biologists Jon Gordon and Frank Ruddle incorporated viral DNA into mouse genomes so that it was passed on to subsequent generations (SN: 9/13/80). The star rodents were called “transgenic” mice. Since then, transgenic and knockout mice, where a single gene is broken or removed, have been developed to mimic and study human diseases from Alzheimer’s to alcoholism to depression and cancer.
3. Bt tobacco and more

In 1987, geneticist Mark Vaeck and colleagues reported that they had genetically engineered tobacco to produce Bt toxins. These toxins, made by the bacterium Bacillus thuringiensis, affect only certain insects, including several common agricultural pests. Pesticides derived from the toxins take time and money to spray, but the new tobacco plant had built-in protection. Estimates suggest that more than 1 billion hectares of Bt crops — corn, cotton, soybeans and more — have been grown since, with no known safety issues for consumers. These crops have improved yields while reducing the need for pesticides. They “are grown on massive scales, in many countries around the world,” says Emma Kovak, a food and agriculture analyst at the Breakthrough Institute, an environmental think tank in Berkeley, Calif. “They’ve had a massive impact.”
4. Flavr Savr tomato

The impact of the Flavr Savr tomato, introduced in 1994, is largely symbolic (SN: 5/28/94). Its genome was modified to block the production of an enzyme responsible for fruit softening, thus keeping the fruit firm longer. High production and distribution costs ultimately doomed the Flavr Savr, but it was the first genetically engineered crop to be approved by the U.S. Food and Drug Administration and to be commercially sold. GM crops have boomed since the Flavr Savr flopped. In 2019, more than 190 million hectares were planted with GM crops. Such crops include potatoes, squash, sugar beets, papayas and corn.
Some people also trace a rise in GMO opposition back to the Flavr Savr tomato, Kovak says. The tomato went through intense safety tests, but people opposed to GMO foods more generally, then and now, point to potential health and environmental risks.
5. Biofortified rice

More than 2 billion people worldwide face micronutrient deficiencies. Traditional breeding and genetic engineering can amp up those nutrients, and rice has been an obvious target. “More than half of the world’s population, including many of those living in poverty, rely on rice for most of their daily calories,” says B.P. Mallikarjuna Swamy, a rice biofortification researcher at the International Rice Research Institute in Los Baños, Philippines.
Golden rice, developed in the late 1990s by a team led by biologists Ingo Potrykus and Peter Beyer, contains genes from a daffodil and a soil bacterium that enable it to produce a precursor to vitamin A. Food safety regulators have approved it in the United States, Australia, Canada and New Zealand, and it was recently approved for commercial use in the Philippines. Despite its promise, though, golden rice has not yet seen widespread adoption due to regulatory hurdles and GMO opposition.
6. AquAdvantage salmon

The FDA approved AquAdvantage salmon for human consumption in 2015 — making the salmon the first GMO animal to be OK’d as human food in the United States. Canada followed in 2016. With a growth hormone gene from Chinook salmon, AquAdvantage salmon reach full size in half the time of traditional farm-raised Atlantic salmon. Fast-growing farmed salmon could have widespread appeal, but there are concerns that if the engineered salmon escape, they could push out wild salmon. For now, AquAdvantage salmon are only trickling into the U.S. supply chain.
7. American chestnut

Some researchers are turning to GMOs for conservation. The American chestnut, which once dominated the eastern seaboard, offers an early example of what such efforts might look like. These “redwoods of the East” were severely reduced by the mid-1900s by a parasitic fungus introduced from imported trees. Historical efforts to develop a blight-resistant chestnut using traditional breeding haven’t panned out, but the Darling chestnut might be the answer. This genetically engineered tree is more resistant to the fungal blight disease thanks to a wheat gene that breaks down the harmful chemical the pathogen produces. The tree has been under review by regulatory agencies since January 2020. After approval, the American Chestnut Research and Restoration Project at the State University of New York College of Environmental Science and Forestry in Syracuse plans to start distributing it to restoration programs and the public.
8. Mosquitoes

Genetically modifying animals that spread disease, including mosquitoes, could save a lot of lives; malaria alone kills hundreds of thousands of people each year. “We’re already using genetically modified mosquitoes for disease control,” says biologist Vanessa Macias of the University of North Texas in Denton. Tests in 2021 in Florida, for example, released male Aedes aegypti mosquitoes genetically engineered so female offspring die before adulthood (SN: 5/14/21). The goal? Reduce the population of insects that spread the Zika and dengue viruses. Modified mosquitoes have also been released in Brazil, the Cayman Islands, Panama and India.
Other research teams are adding genes that make mosquitoes resistant to a pathogen, says Macias, thereby preventing disease spread. And advances in gene editing mean it’s now possible to use what are known as gene drives to spread genetic modifications through entire populations (SN: 12/2/15). Yet open questions remain, including whether it is ethical or wise to transform entire animal populations (SN: 6/3/22). “We’re talking about unknown unknowns,” Macias says.

